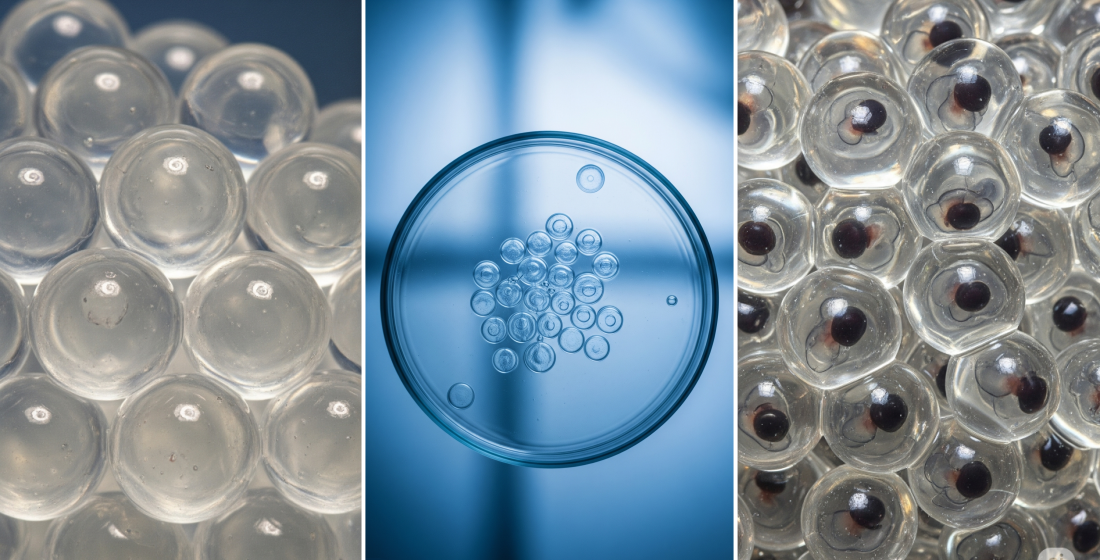
Cryopreservation and Hypothermic Storage of Oocytes and Embryos of Fish and Amphibians. Main Problems and Approaches to their Solution
- Affiliations
-
- 1. Institute of Cell Biophysics RAS FRC PSCBR RAS
- 2. Институт биофизики клетки РАН ФИЦ ПНЦБИ РАН
- Published:
- 2025-03-30
- Keywords:
- cryopreservation, vitrification, hypothermic storage, oocytes, embryos, fish, amphibians, membrane permeability, intracellular ice formation
Abstract
A global decline in animal biodiversity is currently being observed. A significant number of amphibian and fish species are threatened with extinction due to climate change, pollution, habitat degradation, epizootics, and overexploitation. Urgent conservation measures are required to preserve these species, among which genome cryopreservation is one of the most critical. While cryopreservation of sperm in aquatic animals has largely been addressed, there are currently no effective methods for cryopreserving fish and amphibian oocytes and embryos that maintain high viability post-thaw. This review analyzes the structural and physiological characteristics of amphibian oocytes and embryos that impede the development of such methods. These include: multilayered envelopes with low permeability to water and cryoprotectants; a large yolk mass, which restricts cooling rates and hinders cryoprotectant saturation; the presence of osmotically isolated compartments; high sensitivity to cooling. The review summarizes research efforts aimed at overcoming these obstacles to develop technologies for the reversible cryopreservation of these materials. An analysis of hypothermic conservation technologies for fish and amphibian eggs and embryos is also presented.
Full text
Introduction
There is currently a global decline in the biodiversity of our planet's fauna. The results of the analysis carried out by the Species Survival Commission of the International Union for Conservation of Nature indicate that one fifth of currently living vertebrates are threatened with extinction. The largest number of animal species with this status are amphibians (41%, IUCN, 2024 [1]). For fishes, despite the apparent decline in species diversity of marine and freshwater fishes, an accurate assessment is currently impossible, as only 76% of existing species have been assessed to date [1].
Climate change, pollution and habitat degradation, and recent epizootics are the causes of the catastrophically rapid extinction of amphibian species [2,3]. Regarding the decline in fish species diversity, the main threat to marine species is fishing, while freshwater fish are threatened by threats similar to amphibians: pollution of water bodies, disturbance of natural habitat systems, increased epizootics, and overfishing [4,5]. All of the above require urgent measures to conserve vulnerable and endangered amphibian and fish species.
One of the possible methods of amphibian and fish biodiversity conservation is cryopreservation of their genomes. Cryopreservation of sperm from aquatic fauna is a largely settled issue. Successful cryopreservation methods have been developed for more than two hundred species of fish, amphibians and molluscs [6–11]. However, spermatozoa do not allow preservation of the full range of hereditary factors because some of the genetic material, such as mitochondrial DNA, is only transferred through the mother egg.
To date, there are no effective methods for cryopreservation of fish and amphibian eggs [12–15]. There is an urgent need to develop technologies for low-temperature reversible freezing of oocytes and embryos of endangered fish and amphibians. In addition, the development of efficient technology for cryopreservation of fish eggs while maintaining a high level of viability would significantly improve the productivity of domestic fisheries and aquaculture. Many fish species used in aquaculture spawn only once a year. Cryopreservation of fish eggs and embryos would allow more efficient use of fry production capacity, preserve the genomes of valuable fish species and breeds and utilize them as needed.
1. Cryopreservation of fish and amphibian eggs and embryos
1.1. Cryopreservation of fish eggs and embryos
To date, numerous attempts have been made to cryopreserve oocytes and embryos from dozens of fish species [14–16], but experimental results have not been encouraging. In 2005, the survival of 0.07% of Japanese flounder (Paralichthys olivaceus) embryos cryopreserved with propylene glycol and methanol was reported. Out of 30 surviving embryos, 5 successfully hatched [17]. The first relatively successful (50±4% live cells by membrane permeability tests) cryopreservation of early Danio rerio oocytes of developmental stages I and II [18] as well as developmental stage III (vitellogenesis) (20±8% live cells) has been reported [19], but these studies did not evaluate the functionality of surviving cells at the stages of embryo retrieval and hatching. In addition, early oocytes (stages I-III) differ significantly in their properties from mature oocytes and are easier targets for preservation.
A group of researchers from Iran reported successful cryopreservation by vitrification of Persian sturgeon (Acipenser persicus) embryos at the stage of 20-22 somites with a hatching rate of 45% [20]. Unfortunately, this work did not trace the further development of the obtained larvae. Zhang et al. reported survival of 6% of sea bass (Epinephelus moara) embryos cryopreserved by vitrification at the tail bud stage. One third of the surviving embryos developed safely to the hatching stage, but subsequently all of them died within 12-16 days of culturing [21]. The best results were achieved by Khosla et al. in cryopreservation by vitrification of Danio rerio embryos [22]. In this work, they applied direct injection of cryoprotectant into the yolk and an innovative method of ultra-fast heating using a laser. These manipulations resulted in a 40% survival rate of cryopreserved embryos, with 2 of 282 embryos developing to sexual maturity. In reference [23], researchers achieved 32% survival of largemouth bass (Micropterus salmoides) embryos following vitrification by optimizing a cryoprotectant cocktail comprising ethylene glycol (EG), propylene glycol (PG), dimethyl sulfoxide (DMSO), and sucrose.
1.2. Cryopreservation of amphibian eggs and embryos
In contrast to the considerable number of studies on the cryopreservation of fish eggs, very little similar work has been done on amphibians to date. Most studies have focused on the tolerance of amphibian embryos to hypothermia (Rana sylvatica embryos; [24]), cryoprotectants (toad B. viridis embryos, [25,13]). Several papers have been published on the cryopreservation of Xenopus frog stage I and II oocytes, in which factors affecting the formation of intracellular ice were studied [26,128]. Unfortunately, significant progress in cryopreservation of amphibian oocytes, eggs and embryos has not yet been achieved. The best published results on amphibian embryo cryopreservation were obtained by Derakhshan et al [25]. The authors applied modified protocols for vitrification of D. rerio fish embryos to freeze embryos of the toad B. viridis at the blastula and G17 stages and reported recovery of embryos to the neural fold stage after vitrification. However, there has been no continuation of this work and the results have not yet been confirmed by other researchers.
1.3. Major barriers to successful cryopreservation of mature fish and amphibian oocytes
The lack of significant positive results in the cryopreservation of fish and amphibian oocytes and embryos is due to a number of features that distinguish them from vertebrate oocytes and embryos that can be successfully cryopreserved (e.g., mammals). These features, according to the hypothesis, are: 1) the presence of a multilayer system of membranes and shells with low permeability to water and cryoprotectants; 2) the large, compared to mammals, mass of oocytes, which, on the one hand, limits the rate of cooling and heating of these objects, and, on the other hand, provides a low surface area to volume ratio, which makes it difficult to saturate eggs with cryoprotectants; 3) large yolk mass; 4) the presence of osmotically isolated compartments in oocytes and fish embryos [27,13,15]; 5) high sensitivity to cooling [27,15] and 6) abnormally high intracellular ice nucleation temperature compared to mammals [13, 28, 25].
There are a significant number of articles and reviews in the available literature in which authors address the challenges associated with cryopreservation of fish or amphibian oocytes and embryos [15,16,29,7]. However, these papers typically address one or two of the listed problems in detail and are cursory on the others. Below, we have attempted to highlight in detail all the key questions faced by cryobiologists attempting to cryopreserve mature oocytes or embryos from fish and amphibians and analyze the approaches that have been chosen to address these challenges.
It should be noted that early oocytes (stages I-III) differ significantly from mature oocytes in their cryobiological characteristics, and the problems associated with their cryopreservation do not coincide with the problems encountered in the cryopreservation of mature oocytes and embryos. Therefore, from our point of view, consideration of the problems associated with cryopreservation of immature oocytes requires a separate review. In this paper, we do not consider the cryopreservation of immature oocytes.
2. Structure of membranes of fish and amphibian oocytes and embryos. Problems of their permeability
One of the key problems hindering successful cryopreservation of oocytes and embryos of fish and amphibians, as we pointed out above, is the presence of a multilayer system of membranes and envelopes with low permeability to water and cryoprotectants. A large number of studies have been devoted to the morphology, composition and properties of these membranes [30–34].
The egg membranes outside the plasma membrane of fish and amphibian oocytes are of three types in origin: primary (secreted by the oocyte) (e.g., yolk membrane); secondary (secreted outside the oocyte, e.g., by the ovary); and tertiary (secreted by the oviduct, e.g., gelatinous layer). Moreover, in fish oocytes all three types of membranes are present, while in amphibian oocytes only two types are present: primary and tertiary membranes. Unfortunately, in different scientific schools there are significant differences in the names and typology of the shells of fish and amphibians. Many authors try to standardize the terminology in their papers, but it still has significant differences [35–37]. Understanding the structure and composition of oocyte membranes may help to address their low permeability. We will therefore focus on the structure of fish and amphibian egg membranes.
2.1. Structure of fish oocyte (egg) membranes
In cryobiological literature, fish oocyte membranes include the plasma membrane of the oocyte and all membranes overlying this membrane. Among the common features in the structure of the shells of the eggs of different classes of fish (cartilaginous and bony), the presence of all three types of shells: primary, secondary and tertiary should be noted. The primary shells of mature oocytes (eggs) of fish include egg or yolk envelope (cell-free layer), zona radiata, which is a translucent light-refracting membrane formed by the egg itself. This shell is equally spaced apart by pores (tubules) through which the oocyte absorbs nutrients from the space between the plasma membrane of the oocyte and the follicular epithelium, i.e., through which nutrients enter the oocyte (egg) from the ovary. The yolk membrane (also called Vitelline Membrane) is quite tough. It is typically single-layered, composed of several sialylated glycoproteins (usually 2-4) called ZP1, ZP3, ZPax and variants of ZP1 and ZP3 [31, 38–40]. Although there are exceptions, for example, the primary shell in sturgeons consists of two layers, zona radiata interna (ZRI) and zona radiata externa (ZRE) [37].
The secondary membranes of oocytes and fish embryos are often considered as the chorion. However, some authors also refer to the chorion as primary sheaths [33]. The discrepancy between different researchers is due to the fact that in fish, synthesis of envelope components can occur either in the liver, in the oocyte, or in both organs, depending on the species. The chorion is a thick envelope permeated by pore channels with a diameter of ∼200 nm [41] and can consist of several layers (up to three) containing proteins and glycoproteins (called choriogenins) [42, 15, 34]. The composition of the chorion can be altered by environmental and nutritional factors. Valdebenito et al. [33] found that the most common chorion change during embryo development in Atlantic salmon (Salmo salar) is the so-called “soft” chorion [34, 33]. In addition, other chorionic changes have been observed, including perforated and white-stained chorion in Atlantic salmon and coho salmon (Oncorhynchus kisutch).
The tertiary shell of fish, which is a gelatinous layer, as in amphibians, may be present in some species and absent in others [32]. In addition, the tertiary shells of fishes also include the albumen and horny shells (egg capsules) characteristic of cartilaginous fishes [43].
In general, it can be noted that due to the considerable species diversity of fishes, there are significant differences in the number and structure of oocyte and embryo shells in different species. For example, in sturgeons, the number of layers surrounding the oocyte varies from 4 to 5 ones (adhesive layer, alveolar layer, epilayer and zona radiata externa and interna) [44]; the number of micropyle is also different (from 2 to 52) [37]. And among two gudgeon species, Sarcocheilichthys Czerskii (Berg, 1914) (gudgeon) and Sarcoheilichthys Sinensis Bleeker (stag gudgeon), only the primary yolk shell is formed in the stag gudgeon (chorion is absent), while another secondary shell, the chorion, is formed in the gudgeon [45].
2.2. Structure of amphibian oocyte (eggs) membranes
Frog oocytes are characterized by two types of membranes: primary (secreted by the oocyte during development) and tertiary, formed when the mature oocyte passes through the oviduct. Some researchers believe that it is wrong to refer the yolk sheath to the primary, because it is formed from substances secreted not only by the oocyte, but also by follicular cells. The yolk sheath (vitteline membrane/envelope, VE) is composed of proteins and, together with gelatinous layers, surrounds not only the ovulated egg but also embryos at the fragmentation stage [46,36]. The amphibian yolk sheath is composed of 4-6 sialylated glycoproteins called ZP1-4, ZPd and ZPax, which assemble first into fibrils and then into a matrix (egg sheath) surrounding the growing oocytes [38, 40]. Unlike the yolk envelope of fish, it is thicker and multilayered [40].
The most significant species differences in the structure of amphibian eggs are observed in the structure of their tertiary envelolpes - gelatinous shells. For example, the gelatinous shell of oocytes of Xenopus laevis consists of three layers, while in frogs Rana pipiens (Lithobates pipiens) it consists of six layers [40]. In the same amphibian species, researchers can indicate different numbers of gelatinous layers: from 2-3 to 5-6 depending on their method of analysis [45,36]. The composition and properties of the gelatinous layer of eggs of water-laying amphibians and air-laying amphibians differ significantly. For example, in most amphibian species that lay eggs in air, the gelatinous layer has sufficient tensile strength and turgor to allow eggs to retain their shape in air [36]. By composition, gelatin layers are a fibrous glycoprotein structure that serves as a framework to which globular glycoproteins are attached. Each layer has a unique fiber and glycoprotein composition, as well as a different thickness (25 to 200 µm) and structure [47]. The fibers of each layer run at an oblique angle to the fibers in adjacent layers.
2.3. Membrane permeability of oocytes and embryos of amphibians and fish
Oocytes and embryos of fish and amphibians are characterized by low membrane permeability, which may be an evolutionary trait that allows embryos to grow in hypo- or hyperosmotic environments without self-regulatory organs. For example, danio rerio (Danio rerio) embryos have so-called “straightening” membranes that allow water to exit the embryo but prevent it from easily penetrating inside [48].
In the course of studying the membrane permeability of Danio rerio oocytes at different developmental stages for water and cryoprotectants, parameters of fish oocyte membrane permeability such as hydraulic conductivity (Lp) and permeability to solvent (cryoprotectant) (Ps) were investigated for the first time [49]. The authors found that the Lp and Ps values obtained for stage III oocytes were generally lower than those obtained for successfully cryopreserved mammalian oocytes and higher than those obtained for fish embryos and sea urchin eggs. In addition, it has been shown that oocytes at stage III have an osmotically inactive volume of 70%, and the membrane permeability of oocytes at this stage decreases significantly with changing temperature. The authors were not able to estimate the parameters of oocyte membrane permeability at a later stage of development (stage V) because when oocytes were exposed to cryoprotectants, the outer oolemma membrane separated from the inner yolk membrane.
Wang et al. [50] proposed a novel method for real-time assessment of cryoprotectant permeability in fish embryo membranes using electrical impedance spectroscopy. The researchers investigated changes in dielectric permittivity within the low-frequency range (10–103 Hz) and conductivity at high frequencies (104–106 Hz). These measurements were conducted while exposing zebrafish embryos to various concentrations of cryoprotectants: methanol (1.0 M, 2.0 M, and 3.0 M) and DMSO (0.5 M, 1.0 M, and 2.0 M). The findings revealed significant alterations in these electrical characteristics following exposure to methanol and DMSO, particularly at optimal embryo loading levels. The authors concluded that this method, which analyzes the permeability and conductivity of fish embryos, could serve as a valuable new tool for rapid screening of the most effective cryoprotectants. Furthermore, the study demonstrated the potential for quantifying the level of cryoprotectants penetrating fish embryos.
Harvey et al. [51] and others reported a significant effect of the chorion on the permeability of fish embryos to cryoprotectants. Thus, the authors showed that in the presence of the chorion, the isotopically labeled cryoprotectants ([14C] dimethyl sulfoxide, DMSO) and [3H] glycerol, 1M in Fish Ringer) penetrated into embryos extremely slowly and in negligible amounts. Glycerol penetrated the embryo more easily than DMSO, although the degree of glycerol penetration was only about 8% of the expected equilibrium level after 2 h at room temperature; DMSO, in contrast, reached only 2.5% of this level. At the same time, isotope-labeled cryoprotectants penetrated much faster into dechorionized embryos.
It was also shown that the number of embryos hatched after 1-hour exposure to 1.5 and 2 M DMSO with dechorionized embryos was lower than the control. Regarding glycerol, dechorionated embryos exposed to 1 M glycerol for 1 hour at 23°C underwent destruction of periblast cells and separation of blastoderm, and the cryoprotectant could not be removed from the embryos.
The key role of the chorion in the permeability of bony fish embryos (by example of the danio Brachydanio rerio) was also reported by Hagedorn et al. [52, 53]. The authors analyzed the effect of three cryoprotectants, DMSO, propylene glycol and methanol, on their ability to penetrate three- and six-mite embryos (without chorion removal). It was found that 1.5 and 2.0 M DMSO and propylene glycol did not penetrate the embryos (analyzed by osmometric measurements of volume change) [54]. In the same work, the dependence of the permeability of cryoprotectants on developmental stage was noted. In particular, osmometric measurements showed methanol penetration to six-somite danio rerio embryos, but not for three-somite embryos. Permeability to water also depends on the developmental stage of the embryo: it is about the same at the 75% epiboly and trisomic stages, but increases about 2-fold at the six-somite stage [55].
As for amphibians, the membrane permeability of oocytes and embryos in them is even less studied than in fish. Unlike fish, amphibian oocytes and embryos have no chorion and syncytial layer, which, in turn, may contribute to their permeability to water and cryoprotectants. However, there are very few works devoted to the study of this feature. For example, there are some publications on the permeability of oocytes and amphibian embryos to water and urea [56–58] reported extremely low permeability of the oocyte plasmalemma to water. Lau et al. [57] studying the permeability of oocytes of tailless amphibians of the genus Rana, found their permeability to urea. It has also been shown that decreased permeability of embryonic membranes correlates with embryonic maturation, which is designed to prepare the embryo for a “hostile environment”. The permeability of oocytes and embryos of fish and amphibians at different stages of development may also be affected by aquaporins, the presence of which in these taxa, as well as their role in fluid movement in gametes, is still poorly understood [26, 59]. Despite similarities in the structure of early fish and amphibian embryos, the differences do not allow to directly extrapolate the developing permeability models between taxa [13].
2.4. Ways to increase membrane permeability of oocytes and embryos of fish and amphibians
To overcome the problem of low permeability of oocytes and embryos of fish and amphibians, a number of physicochemical methods have been applied by various researchers. Let us highlight some of them.
2.4.1. Ultrasound
Ultrasound is considered as one of the promising methods to increase the permeability of fish embryos. There are a considerable number of publications devoted to the study of its effect on the permeability of embryos of this taxon. Wang et al. [60] studied the effect of ultrasound on the permeability of danio rerio embryos to methanol. Embryos at the 50% epiboly stage, after pre-exposure for 25 min with 2 M methanol, were treated with ultrasound for 5 min at 22°C at different frequency combinations, varying the voltage applied to the ultrasonic transducer from 50 to 175 V. The result was evaluated by the change in dielectric permittivity of the embryos, which was measured by impedance spectroscopy. The authors found a tendency for the dielectric constant to increase with increasing voltage up to 175 V in the low impedance frequency range of 10-103 Hz, from which it was concluded that the penetration of methanol into the embryos increased after ultrasonic treatment in the above regime. Unfortunately, the paper lacks information that would allow one to calculate the intensity of ultrasound exposure in traditional units (W/cm²).
Silax and Barth [61] also investigated the effect of ultrasound on methanol (MeOH) penetration into danio rerio (D. rerio) embryos of three types (dechorionized, with soft chorion, and intact embryos) and three developmental stages (90% epiboly, bud and 4-somites stages). In their experiments they used 47 kHz and 480 V ultrasound for 1 min as well as 1, 2, or 3 min of incubation in methanol (20, 30, or 40%). High-performance liquid chromatography (HPLC) was used to measure methanol levels in treated embryos. A maximum methanol permeation of 85.3 ± 8.1 μmol was shown on embryos with soft chorion at the 90% epiboly stage when treated with ultrasound. However, the level achieved was well below the level required for their vitrification.
Rahman et al. [62] investigated the effect of ultrasound on the extent of cryoprotectant uptake by embryos of Japanese whiting Sillago jaropisana at two developmental stages (somites and tail elongation). The authors showed that the cryoprotectant DMSO content in ultrasound-treated embryos at somites and tail elongation stages increased by 58-191% and 27-123%, respectively, compared to control samples exposed to DMSO without ultrasound. In addition, pre-exposure to DMSO prior to ultrasound treatment increased cryoprotectant uptake by an additional 36% without impairing survival. Embryos were also found to tolerate well ultrasound intensities up to 37.5 W/cm2 for 3 min, but died at 50 W/cm2. It was noted that after ultrasound treatment of embryos at the somite stage in 10 and 20% DMSO solutions, the percentage of hatching was quite high and amounted to 65-86%. Increasing the concentration of DMSO to 30% significantly decreased the hatching percentage. The stage of embryo development during ultrasound treatment also affected the subsequent development of embryos. Thus, the authors noted that under the same ultrasound treatment conditions, embryos with tail elongation had a lower survival rate than embryos at the somite stage.
As for amphibians, there are not many studies on the effect of ultrasound on the membrane permeability of oocytes and embryos. Melnikova et al., [63] showed that the permeability of the embryonic membrane of the tailless amphibian gray toad Bufo bufo and the grass frog Rana temporaria for the slowly permeable fluorochromes ANS (anilinonaphthalene sulfonic acid), FDA (fluorescein diacetate) and fluorescein increases under the action of high-frequency ultrasound. Changes in membrane permeability of amphibian embryos under the action of ultrasound were also reported by Gakhova et al. [64,65]. The authors reported an increase in the permeability of R. temporaria embryos at the blastula stage as a result of exposure to ultrasound with a generation frequency of 0.88 MHz and intensity of 0.5-0.7 W/cm2. Changes in permeability were assessed by FDA staining of embryos. After ultrasound treatment and fluorochromization of embryos with FDA, germ cells fluoresced in contrast to the control (without ultrasound treatment). This indicated an increase in blastula membrane permeability, and the embryos continued to develop normally after ultrasound exposure.
To determine the optimal range of ultrasound exposure that does not adversely affect the survival and development of grass frog embryos, the effect of continuous and modulated ultrasound of therapeutic intensity range was analyzed [66, 67]. The authors confirmed that when continuous ultrasound was used, the highest survival rate up to the hatching stage was observed in embryos taken into the experiment at the blastula stage and treated for 5 minutes with continuous ultrasound of low intensity (0.05 W/cm2) at a generation frequency of 0.88 MHz. Exposure to ultrasound with an intensity of 1.0 W/cm2 decreased the number of embryos developing before hatching [66]. The use of modulated ultrasound, involving treatment of embryos for 5 min by ultrasound with a generation frequency of 0.88 MHz, ultrasound intensity of 0.4 W/cm2, modulation frequency of 40 Hz or 98 Hz, provided the maximum percentage of embryos that reached the hatching stage [67].
2.4.2. Introduction of mRNA aquaporins
Hagedorn et al. [48] proposed the introduction of aquaporin-3 water channel protein mRNA as a solution to the problem of low permeability in fish oocytes and embryos and demonstrated an increase in membrane permeability to water and cryoprotectant. Radioactively labeled propylene glycol was shown to diffuse into the yolk. The authors concluded that this method delivered sufficient cryoprotectant to aquaporin-3-expressing Danio rerio embryos for successful cryopreservation. The introduction of aquaporin-3 mRNA was also effective in improving the permeability of X. laevis oocytes. In experiments by Yamaji et al. [68], ectopic expression of aquaporin-3 channel in X. laevis oocytes increased the permeability of the oocyte plasma membrane to ethylene glycol, propylene glycol, and glycerol.
2.4.3. Laser treatment
Kohli et al. [69] reported the ability to enlarge pores and introduce foreign material into developing fish (zebrafish) embryos using femtosecond (fs) laser pulses. As a result, a fluorescent reporter molecule (fluorescein isothiocyanate) conjugated to streptavidin, quantum dots, or DNA (Simian-CMV-EGFP) was successfully injected into chorionized and dechorionized embryos of danio fish. The authors noted that the survival rates of dechorionized and chorionized embryos laser manipulated at the pectoral fin stage were 89% and 100%, respectively. It is possible that this method will also be effective in increasing the permeability of oocytes and embryos of fish and amphibians to cryoprotectants.
2.4.4. Hydrostatic pressure
Some researchers used hydrostatic pressure to increase the permeability of fish and amphibian oocytes and embryos to cryoprotectants [70]. For example, these authors showed that the use of 50 atm hydrostatic pressure combined with treatment of 8-cell medaka (Oryzias latipes) embryos for 3 min with a 1 M trehalose solution significantly increased the DMSO concentration inside cells compared to the control. In addition, the stage at which hydrostatic pressure was applied to oocytes and embryos was found to be significant. At the unfertilized egg stage, application of hydrostatic pressure also promoted DMSO uptake, but oocytes rapidly lost viability; at the eye embryo stage, application of hydrostatic pressure was also ineffective.
2.4.5. Electroporation
The use of this method also helped to increase the permeability of embryos to cryoprotectants. Rahman et al. [71] found that electroporation of putassu (Sillago japonica) embryos at 300 V in 10%, 20% or 30% DMSO promoted the uptake of cryoprotectant by the embryos and its content in treated embryos was 10, 30 and 78 mM without loss of survival. However, treatment of embryos with higher voltages, along with increased DMSO uptake (up to 84 mM), decreased the embryo survival. Although pre-exposure of embryos to 10% DMSO for 20 min before electroporation increased DMSO uptake (up to 116 mM), the authors concluded that the DMSO concentrations obtained by electroporation were insufficient to prevent ice formation in embryos during freezing and thawing.
2.4.6. Osmotic and chemical treatments
Application of solutions with high osmolarity can also temporarily increase the permeability of fish embryo membranes. For example, Rahman et al. [72] showed that treatment of Japanese putassu S. japonica at the somite and tail elongation stages with 1 M trehalose for 3 min before incubation with DMSO increased cryoprotectant uptake by 45% without significant effect on embryo viability, while treatment with the enzyme pronase (2-6 mg/mL) did not have such a marked effect on DMSO permeability. In addition, the authors found that the DMSO content of embryos increased by 143-170% in the presence of 0.25 M MgCl2 and 0.125 M CaCl2. However, the best combination was the treatment with trehalose and MgCl2, which was even more effective in stimulating DMSO penetration (191 % compared to untreated embryos). Thus, the authors concluded that the use of trehalose as a dehydrating agent and MgCl2/CaCl2 as a carrier during incubation with cryoprotectant promotes increased cryoprotectant uptake and may be a promising method of preparing fish embryos for subsequent cryopreservation.
2.4.7. Microinjections
Several authors showed the promise of using microinjections to increase cryoprotectant content in fish and amphibian oocytes and embryos for cryopreservation [73–75]. The use of single or combined microinjection of cryoprotectants into Japanese putassu S. japonica embryos reduced the nucleation temperature and increased the embryos' resistance to chilling; at different developmental stages the tolerance of embryos to amounts of injected cryoprotectants varied from 2.1 to 15.6 nL [75]. It was also shown that microinjections allowed for delivering high concentrations of cryoprotectant into the yolk sac without deleterious effects on the fish embryo, but they failed to provide a significant level of protection to the whole embryo from cold injury [74]. The use of microinjection does not always prove to be safe for oocytes. For example, Jevtich et al. [76] found that the microinjection procedure itself causes significant damage to amphibian oocytes and as a result the microinjected Xenopus laevis oocytes were not fertilized. The conditions of microinjection application, including the injection site, require further experimental optimization.
3. Yolk
3.1. Yolk structure
The presence of large amounts of yolk in fish and amphibian eggs and embryos is the next key problem preventing their successful cryopreservation [77]. Both fish and amphibian oocytes are of the telolecytic type, and only some of them have oocytes of the mesolecytic type, but even in these, the amount of yolk is quite high [78–80]. From the point of view of cryobiology, it is important to know that fish oocytes are subdivided into two types: isolated-yolk oocytes (with subsequent meroblastic fragmentation) and non-isolated-yolk oocytes (with holoblastic fragmentation) [80]. Isolated-yolk eggs have a syncytial layer around the yolk, which has a great influence on the interaction of the embryo with cryoprotectants and on the movement of water between different compartments of the embryo. Non-isolated-yolk eggs do not have this structure. The eggs of most bony and cartilaginous fishes belong to the isolated-yolk eggs, while the eggs of sturgeons (Acipenseridae), hornbills (Ceratodontiformes), and some other fishes belong to the non-isolated-yolk eggs. The eggs of amphibians are similar in structure and mode of fragmentation to the non-isolated-yolk eggs of fish [81,78,79]. Therefore, we considered it appropriate to describe the yolk structure in fish with non-isolated-yolk eggs and amphibians in one section.
Amphibian and fish eggs with non-isolated-yolk are characterized by dispersed distribution of yolk mass with concentration in the vegetative part of the egg. The structural components of the yolk are yolk plates and fat droplets. In some cases, single, isolated glycogen granules can be observed in the yolk [78, 79]. The yolk plates consist of a lipoprotein core, in which pseudocrystalline structures are often (but not necessarily) observed, and a membrane surrounding the core. The size of the yolk plates varies greatly among species and depending on location in the egg, but is most commonly 4-15 µm [78]. The yolk plate nucleus contains predominantly lipoproteins, with the weight fraction of proteins usually predominating over or about equal to lipids. Proteins are represented by lipovitellin and phosvitin or similar substances; lipids are phospholipids, and triglycerides may be present in very small amounts. The yolk plate envelope consists mainly of mucopolysaccharides [78, 79]. Fat droplets are located among the yolk plate, and their number and size vary among species. Unlike yolk plates, fat droplets contain mainly triglycerides but may also contain wax esters, carotenoids and proteins [78, 82]. During egg fragmentation, yolk components are distributed among dividing blastomeres, concentrating mainly in the larger cells of the vegetative pole [80].
The yolk in fish with isolated-yolk eggs may retain a dispersed structure similar to the structure of non-isolated-yolk eggs, but may also merge into a homogeneous mass, partially or completely. In particular, in salmonid, flatfish, and cod fishes, yolk granules merge into a single mass during egg maturation, whereas in carp, finfish, and catfish they retain a granular structure [80, 83, 84]. Fat droplets can also coalesce into one or more large fat droplets during early embryonic or larval development [78, 85]. The chemical composition of the yolk in fish with isolated-yolk eggs is not fundamentally different from that of non-isolated-yolk eggs.
The most characteristic feature of embryonic development of bony fishes with isolated-yolk eggs is the formation of the extraembryonic yolk sac. A feature of the extraembryonic yolk sac is the presence of the yolk syncytium or syncytial layer, also sometimes called the syncytial membrane, a specialized tissue responsible for the utilization and digestion of yolk by the embryo. The presumed yolk syncytium, the periblast, is found in the fertilized egg of bony fishes as early as at the unicellular stage [86]. The complete syncytial layer is formed at the blastula stage [83, 87]. From the point of view of cryobiology, the most important feature of the syncytial layer is its extremely low permeability to water and cryoprotectants [52, 88, 89, 90].
3.2. Effect of yolk on the sensitivity of oocytes and embryos of fish and amphibians to cooling
Mature oocytes and embryos of fish and amphibians are characterized by low tolerance to low temperatures. Even short-term decreases in temperature of more than 10-15 degrees below the physiological one result in developmental pathologies or death [24, 54, 91–96]. Sensitivity to chilling is often attributed to the presence of large amounts of lipid-rich yolk [15, 97–99]. Indeed, partial removal of yolk increases the tolerance of Danio rerio embryos to cold shock [92, 100]. It was also found that primary germ cells, retained in the whole embryo, recovered better and showed higher viability if the yolk was partially removed from the embryo before the vitrification procedure [101, 102]. Unfortunately, the above data were obtained on only one species, Danio rerio, which does not allow us to draw fundamental conclusions about the negative effect of yolk on the cold tolerance of fish eggs. Nevertheless, it is possible to draw certain parallels with mammalian embryos.
Despite the fact that mammalian embryos in general have much less yolk compared to fish and amphibians, they also contain yolk inclusions. It was found out that yolk-rich oocytes and mammalian embryos are less tolerant to refrigeration and cryopreservation compared to yolk-depleted embryos [103, 104]. For example, yolk-rich pig oocytes [105] and ferret embryos [106] are difficult to cryopreserve, whereas cryopreservation of mouse embryos containing very little yolk is well established. The hypothesis about the negative effect of yolk on the tolerance of embryos to cooling and freezing was also supported by Abe et al. [107]. A large number and larger size of lipid inclusions were shown to correlate with low cryotolerance of bovine embryos [107]. Based on the parallels of the effect of yolk on the cold tolerance of embryos and oocytes in mammals and the fish Danio rerio, it can be assumed that this pattern is universal.
It is noteworthy that the sensitivity of embryos to cooling depends not only on the mass fraction of yolk inclusions, but also on the composition of lipids. For example, despite the fact that cat embryos are rich in yolk, they have quite high survival rates after cryopreservation [108, 103]. This is explained by the fact that cat embryos contain many unsaturated fatty acids, due to which the phase transitions in lipids (liquid crystal/gel) occur at lower temperatures [109], thus contributing to the embryos' resistance to cold shock and freezing [104].
In spite of the fact that the mechanism of cryodamage of lipid membranes is fairly well understood [110, 111], in case of yolk-rich oocytes and embryos it remains not completely clear yet [99]. It is known that the liquid crystal/gel phase transition in the lipid bilayer of cell membranes plays a key role in the development of cold shock. The cold damages the cell membrane integrity due to changes in the packing of molecules during the phase transition, it causes the loss of a part of membrane proteins and changes in semipermeability properties, which disrupts cellular homeostasis [111]. Since lipids in yolk plates and yolk lipid droplets are located in the inner layers - in the core of yolk plates or lipid droplets, phase transitions of lipids in yolk cannot disturb the homeostasis of these structures, and therefore the mechanism of this damaging effect should be different from that one of the cell damage caused by the disruption of the cell membrane integrity. It can be assumed that cooling disrupts protein-lipid interactions in yolk lipoproteins, leading to their partial destruction [112]. It can also be hypothesized that due to phase transitions large lipid inclusions can significantly change their volume and configuration, resulting in the damage to the yolk plate shells and/or lipid droplets and surrounding structures. Indeed, transmission electron microscopy studies on ferret embryos showed that multiple discontinuities appear in the electron-dense rim of cytoplasmic lipid droplets after vitrification [106]. The yolk inclusions in fish and amphibians are much larger than in mammals, and accordingly, the negative phenomena resulting from phase transitions in lipids may be more pronounced and lead to more serious consequences.
3.3. Increasing the chilling tolerance of oocytes and fish embryos through yolk manipulation
3.3.1. Use of eggs at developmental stages with low yolk content
One fairly obvious way to overcome the negative effects of large yolk masses is to handle embryos and oocytes at a time when they contain minimal yolk inclusions. These can be early oocytes before the beginning of active vitellogenesis, or embryos at later stages of development when the yolk has already been partially utilized. Early oocytes are easier targets for cryopreservation not only because of their low yolk content, but also due to other factors (small size, higher permeability of the membranes). However, working with early oocytes requires rather complex techniques for their cultivation and maturation in vitro, which imposes certain limitations on this direction. As for embryos at later stages of development, studies do not support their higher cold tolerance [54, 91, 94, 95, 100, 113, 114]. This appears to be due to the more complex organization of embryos at later development stages: their tissues and cells exhibit a much higher degree of differentiation, and at this stage they form complex organs. Different types of cells and tissues have different sensitivity to cold shock, osmotic and oxidative stresses caused by cold; they demonstrate different resistance to the decrease in metabolism observed in low temperatures. Apparently, all these factors make embryos at later stages of development more sensitive to cooling also in those cases when the influence of decreasing yolk masses drops down.
3.3.2. Vitrification
Some authors believe that vitrification technology may offer hope for successful cryopreservation of fish and amphibian eggs [13, 15, 21, 24, 90, 99]. Indeed, rapid and ultrafast freezing, which promotes vitrification of the biological object, is able to prevent not only ice crystal formation but also temperature-induced conformational changes of lipid inclusions. However, it is known that phase rearrangements in lipids start at much higher temperatures compared to water crystallization and have a very wide temperature range [111, 115, 116]. Many experimental vitrification protocols involve a slow cooling of the bio-object to near-zero temperature (0°C to 6-8°C), followed by its immersion directly into liquid nitrogen and rapid freezing [20, 21, 114]. Such a protocol cannot prevent lipid phase transitions, which in many species, especially warm-water ones, begin as early as at 15-20°C [115, 116]. For successful vitrification of mature oocytes and embryos of warm-water species, a better understanding of the behavior of yolk components during cooling is needed.
3.3.3. Partial yolk removal
Partial yolk removal by mechanical means is currently considered to be the most promising approach to improve the cryostability of yolk-rich oocytes and embryos. Fish embryos tolerate removal of up to 2/3 of the yolk without adverse effects [92, 117]. Several studies showed that this manipulation reduces the sensitivity of fish embryos to cold shock [100] and freezing [89, 101, 102]. Although this method has not been successful in cryopreserving fish embryos to date, it may be useful in combination with other approaches.
3.4. Syntycial Layer and Ways to overcome the low permeability of the synticial layer
As mentioned above, the syntycial layer is a special structure surrounding the yolk of isolated-yolk eggs. For bony fishes, the presence of the syntycial layer is considered a major obstacle for successful embryo cryopreservation, as it plays a serious limiting role for the penetration of cryoprotectants into the yolk layers [13, 22, 118]. As early as in the 1990s, it was shown that even if cryoprotectants pass through embryonic membranes into the blastoderm, they do not penetrate into the yolk of bony fish thus preventing the achievement of the necessary levels of cryoprotectants for successful cryopreservation [53, 55, 88].
Due to the under-saturation of cryoprotectants, the yolk becomes the main site of ice crystal nucleation, which leads to damage of the whole embryo [55]. The synticial layer has a very limited permeability not only to cryoprotectants but also to water. It limits the outflow of water from the yolk during slow cryopreservation, and prevents the desired level of dehydration [55, 88]. In particular, the permeability of DMSO to blastoderm and yolk differs by three orders of magnitude: ≤ 5 × 10-6 and 1.5 × 10-3 cm/min, respectively [88]. The synticial layer itself is also damaged by freezing. The ultrastructural studies showed that after vitrification the yolk-synticial layer is damaged much greater than the blastoderm and yolk itself [88].
Thus, the low permeability of the synticial layer to cryoprotectants prevents successful vitrification, and its low permeability to water prevents achieving the necessary level of dehydration during slow freezing [55, 88]. It means that the synticial layer is a “stumbling block” in the cryopreservation of fish embryos by both the slow (classical) method and by vitrification. The following methods address the challenge of low permeability within the syncytial layer.
3.4.1. Methanol
Methanol has the highest permeability among all cryoprotectants. It is able to pass not only through cell membranes but also through human skin [119]. Not surprisingly, experts developing methods for cryopreservation of fish embryos and oocytes have turned their attention to methanol. Using magnetic resonance microscopy and magnetic resonance spectroscopy, Hagedorn et al. demonstrated that methanol, unlike cryoprotectants such as DMSO and propylene glycol, is able to pass through the synticial layer of the Danio rerio embryo at the stages of three and six somites [52, 53]. Liu et al. demonstrated that exposure of dechoreinized danio rerio embryos to 2M methanol solution significantly reduced the freezing point of intraembryonic water [89]. Unfortunately, the same authors showed that the protective properties of methanol are insufficient for successful cryopreservation of fish embryos.
3.4.2. Ultrasound
The effect of ultrasound on the permeability of fish and amphibian embryonic membranes is described in Section 2.4.1. It is believed that ultrasound can also increase not only the permeability of embryonic envelopes and cell membranes, but also the permeability of the synticial layer [120]. Unfortunately, current research into the effect of ultrasound on the rate of cryoprotectant penetration into fish embryos cannot clearly confirm or refuse this assumption because no differentiation between yolk and blastoderm cryoprotectant saturation was carried out [60–62, 121].
3.4.3. Introduction of mRNA aquaporins
The permeability of fish embryos can be increased by introducing mRNA for the aquaporin-3 protein. Hagedorn et al. showed that expression of aquaporin-3 opens the access for the cryoprotectant propylene glycol not only to the blastoderm but also to the yolk. Thus, it can be considered that artificially introduced aquaporin mRNA can increase the permeability of the synticial layer for cryoprotectants [48]. Unfortunately, there is a paucity of research in this domain.
3.4.4. Injections.
Direct microinjection of protective substances into the yolk is considered to be the most promising method to date [22, 90]. The Danio rerio embryo can tolerate the injection of a solution of about 7-10 nL [90, 118, 122] or even 30 nL [118] into the yolk without fatal consequences. The injection of cryoprotectant solutions such as DMSO [118], propylene glycol [90, 118], methanol [122], sucrose [122] was tested. Similar experiments were carried out on another object, Japanese putassu (Sillago japonica) embryos at several developmental stages. The putassu embryos tolerated well the injection of propylene glycol solution into the yolk. The authors noted that after injection of cryoprotectant simultaneously into the yolk and perivitelline space, the cooling tolerance of the putassu embryos was significantly increased [75].
Despite the encouraging results of the above-mentioned works, the method of direct injection of cryoprotectant into the yolk sac did not help to obtain fully viable and capable of development frozen-thawed fish embryos. The situation changed after the experiments of Khosla et al. [22, 90]. These researchers combined the method of injecting a cryoprotectant directly into the yolk with heating the frozen biomaterial with an infrared laser. By combining these two approaches, they were able to achieve the recovery of developmentally capable danio rerio embryos for the first time [22, 90]. These experiments are described in more detail in Section 5.1.2.
4. High-temperature nucleation of intracellular ice
The most important factor determining the success of cryopreservation is to prevent the formation of intracellular ice, which has lethal effects on the living cell [123]. The nucleation of ice crystals at decreasing temperature is a stochastic process, but nevertheless obeying certain regularities. During cooling of mammalian embryos and oocytes, nucleation of intracellular ice always occurs at a lower temperature than nucleation in the external solution. For example, in mouse oocytes frozen in the absence of cryoprotectants, the average temperature of intracellular ice nucleation occurs at about -14°C, while in the external solution ice appears already at -(2-4)°C [124, 125].
The addition of cryoprotectants enhances the difference in the nucleation temperature of extracellular and intracellular ice. For example, in the presence of 1-1.5 mol of glycerol or ethylene glycol, extracellular crystallization occurs at temperatures between -3.9°С and -7.8 °C, while the average nucleation temperature of intracellular ice (assuming sufficiently rapid freezing to prevent redistribution of cryoprotectants between the extra- and intracellular environment) is -41°C [124, 125]. A similar pattern is observed during cooling of bovine oocytes [126]. It is this property that allows slow (classical) freezing of mammalian embryos. When using this technology, the formation of extracellular ice increases the osmolality of the residual unfrozen solution, which, in turn, causes water outflow from the cell, its dehydration, an increase in the intracellular concentration of cryoprotectant and, as a consequence, leads to vitrification of the intracellular space without the formation of ice crystals [124, 127].
However, studies of crystal nucleation patterns outside and inside fish and amphibian eggs show a different picture. When cooling embryos of Danio rerio fish [28,89] or spurred frog oocytes [26,128], intracellular ice nucleation occurs almost simultaneously with the appearance of ice in the external solution, i.e., at a relatively high temperature. This is not a unique situation: a similar pattern is observed during freezing of starfish oocytes [129], whose eggs are similar in size and structure to fish and amphibian eggs. The study of the ice nucleation process on the model of danio-rerio embryo showed that when the temperature of ice formation in the external solution is increased by artificial heating, the temperature of intracellular ice crystal nucleation increases simultaneously. In the vast majority of cases, intracellular nucleation occurs at the moment when the ice-forming front of the external solution reaches the embryo [24]. Direct observations of ice formation on a cryomicroscope showed that in spurred frog oocytes, nucleation always occurs at the periphery and then rapidly spreads to the entire oocyte [128]. These results made it possible to conclude that unlike mammalian embryos, in which oocytes can be cryopreserved slowly without the initiation of endogenous ice nucleation, in fish and amphibians this cannot be achieved due to the initiation of intracellular nucleation by external ice crystals at the early stage of freezing. The mechanism of this phenomenon is not fully understood yet. Hagedorn et al., [24] suggested that there are three possibilities (hypotheses) as to how exactly extracellular ice can initiate intracellular nucleation.
The first hypothesis is that envelopes (including the plasma membrane and egg shells) have one or more pores of sufficient diameter to allow ice crystals to grow from the outer solution into the interior. Indeed, the outer membranes of fish oocytes have channels, and Mazur estimated that ice can sprout into pores up to one angstrom in diameter [130]. At the same time, the presence of large pores in the egg envelopes of fish and amphibians, sufficient for ice grow, contradicts the low permeability of these structures for water and cryoprotectants.
The second hypothesis is that when external ice comes in contact with the surface of the embryo, membranes undergo physical deformations resulting in the appearance of sufficiently large defects, or the size of existing pores increases thus allowing growing ice crystals to enter inner parts of the embryo.
The third hypothesis is that when external ice comes in contact with the outer membrane, the structure of the latter undergoes changes, resulting in the formation of ice nucleation sites on its inner side [131].
The latter two hypotheses have a right to exist, but have not been confirmed experimentally yet. Nevertheless, it can be assumed that the nucleation of intracellular ice in amphibian and fish embryos occurs under the influence of external ice, at least in the species studied in this respect.
This state of affairs, as rightly noted by a number of authors [13, 24, 99], makes it futile to freeze fish and amphibian eggs using the slow method, since the key point of this technology lies in the slow nucleation of intracellular ice compared to the external solution [124, 125]. When extra- and intracellular ice forms simultaneously, this mechanism of additional cell dehydration becomes inoperable [13, 24, 99]
4.1. Possible solutions
This property of embryos and fish naturally leads to the conclusion that the only possible method of freezing mature oocytes and embryos of fish and amphibians may be vitrification [13,99], where extra- and intracellular ice nucleation is suppressed. Almost all current research on freezing oocytes or embryos of fish and amphibians lies in this area [13–15, 22, 90].
High concentrations of cryoprotectants, which are extremely toxic to fish and amphibian eggs, are used to prevent ice nucleation both in the external solution and inside embryos or oocytes. An alternative method of eliminating crystallization of extraembryonic water is to create an anhydrous freezing environment. This is the path followed by the group of Tikhomirov et al. Caspian sturgeon (Acipenser persicus) eggs were immersed in oil and frozen using the liquid nitrogen immersion method. The authors claim that using this method they were able to obtain from 50 to 85% of live oocytes after the freeze-thaw procedure [132, 133, 134]. However, there is still no experimental confirmation of these results by other authors.
5. Challenges associated with the large size of mature oocytes and embryos of fish and amphibians
Most researchers involved in the cryopreservation of fish and amphibian eggs cite the size of mature oocytes or embryos of these taxa as one of the key obstacles to successful cryopreservation [11, 13, 15, 90, 99, 127, 135, 136]. Indeed, in both fish and amphibians, unfertilized eggs or embryos are relatively big: from 0.5-20 mm in diameter in fish [137], 0.5-12 mm in tailless amphibians, 1-10 mm in tailed amphibians, and up to 42 mm in diameter in some species of legless amphibians [11, 99].
Mature oocytes and early animal embryos, for which successful cryopreservation protocols have been developed, are much smaller. For example, the diameter of a compact mouse morula (including the shiny shell) is 90 μm [81], bovine blastula is 150-200 μm [138], and bovine oocytes are 110-130 μm [139]. The diameter of a mature human oocyte is 150 µm [140]. Among aquatic species, successful cryopreservation of oocytes of the giant oyster (Crassos treagigas) is known [141, 142]; the oocyte size of this species is also relatively small, being approximately 50-60 μm in diameter [143].
The big size of mature oocytes and embryos of fish and amphibians generates three major problems that complicate the cryopreservation process [11]. The first problem is a low surface area to volume ratio, which slows down the saturation/washout process of the eggs as they interact with cryoprotectants. Also, less quick will be the rate of cell outflow and subsequent rehydration during slow cryopreservation, making cryopreservation by this method much more difficult [99, 127]. The low surface area to volume ratio is exacerbated by the low permeability of the egg membranes of mature oocytes and embryos to water and cryoprotectants. This aspect of cryopreservation of fish and amphibian oocytes and embryos is discussed in more detail in Section 2.3.
The second problem is the inability to achieve high cooling/heating rates using conventional cooling/heating. For classical (slow) freezing this is not a significant factor, but for vitrification, which is currently recognized as a more promising approach for the cryopreservation of fish and amphibian eggs [13, 90, 99, 97, 135] this limitation is critical.
Cooling during vitrification should occur faster than water crystallization, while the rate of thawing should prevent devitrification. These rates depend on the total amount and type of cryoprotectants used. The higher is the concentration of cryoprotectants, the lower are critical freezing and thawing rates required for successful vitrification. Fish and amphibian oocytes and embryos are quite sensitive to cryoprotectants, and their maximum concentration that does not cause a significant decrease in embryo and oocyte viability usually lies between 2-3 Mol/L [24, 62, 72, 90, 98, 121]. Cooling rates on the order of 20,000°C/min and thawing rates on the order of 10,000,000°C/min are believed to be necessary for successful vitrification of fish oocytes/embryos [144]. By freezing in quartz capillaries, in the Cryotop system, in Cryo-loops or in straws with an elongated tip with a diameter of about 0.9 mm (Open Pulled Straw system), the required freezing rates can be achieved, but for technical reasons only the Cryotop system is suitable for fish and amphibian eggs [144]. Khosla et al. showed that embryos of danio rerio fish can be cooled with this system at the required rate [22, 90, 144]. However, the required thawing rate for such a large object using conventional convection heating remains elusive [22, 90, 144].
There is a third problem arising from the large size of fish and amphibian oocytes and embryos that is rarely addressed in reviews of cryopreservation of these objects. Oocytes and embryos of most species of these two classes of animals are large enough for low-temperature cracking to be a significant factor. Cracking is the destruction of solidified cryopreserved objects. This phenomenon is primarily induced by thermomechanical stresses stemming from two principal factors: uneven cooling/heating of the external and internal regions of the object and/or the difference in the temperature expansion/contraction coefficients of frozen biological structures and aqueous solutions in the temperature zone below the point of complete transition of the liquid phase to the solid state [145–149]. Significant cracking can cause permanent damage to the frozen object [150, 151]. Minor cracking is dangerous because the cracks can become ice nucleation sites and provoke devitrification of the object upon thawing [152]. Cracking can occur during both slow and rapid (vitrification) freezing, with the latter usually being more pronounced. The cracking onset temperature depends on the medium composition and cooling rate [147, 148, 151, 153, 154].
For vitrifying solutions, thermomechanical stresses may begin to form at temperatures slightly above the vitrification temperature, but the solution cracking begins at temperatures approximately 5-10 degrees lower [146, 155]. The size of ice microparticles formed as a result of cracking also varies depending on the composition of the media and the cooling rate. It most often lies between 0.1-0.5 mm, which is smaller than the embryo diameter of most fish and amphibians [151, 154]. Unfortunately, this process is practically unstudied with respect to oocytes and embryos of fish and amphibians.
5.1. Ways to solve the problem of the large size of mature oocytes and fish and amphibian embryos for cryopreservation
5.1.1. Increasing the rate of saturation and washing of cryoprotectants
Since we cannot change the size of mature fish and amphibian oocytes, it is possible to increase the rate of saturation and washing of cryoprotectants only by increasing the permeability of the embryonic membranes. Studies in this direction are discussed in detail in Section 2.4. In addition, it is possible to focus on cryopreservation of immature oocytes, the size of which is much smaller, but this area of interest has its own difficulties. It is beyond the scope of this review to describe in detail the research on cryopreservation of immature oocytes.
5.1.2. Increasing the rate of freezing and thawing of oocytes/embryos.
Since, as mentioned above, the cooling rates required for vitrification of fish eggs are achievable, a key objective is to increase the rate of warming of the vitrified objects. The rate of thawing can be increased by replacing (or supplementing) standard thawing by microwave heating [156], radiofrequency heating [149], or irradiation with infrared light (IR heating) [157, 158].
By themselves, these methods are either insufficient or cause undesirable temperature gradients due to the uneven absorption of irradiation energy by different cell structures [149]. However, their efficiency can be greatly improved by preloading the vitrified object with particles that absorb radiation energy more efficiently and convert it into thermal energy. Such particles are called heat receivers. For microwave irradiation and radiofrequency electromagnetic field, magnetic particles, most often iron oxide nanoparticles [159], and for infrared irradiation these heat receivers can be either soot microparticles or gold or titanium nanorods [22, 90, 160].
Microwave heating and radiofrequency heating have not yet found application in the vitrification of oocytes and embryos of aquatic species, but the use of IR heating is not only being actively investigated, but has also led to noteworthy results. In 2014, Masur et al., showed that a 0.1 µL droplet containing soot microparticles can be heated at a rate of about 1 million degrees per minute using pulsed irradiation with a laser 1064 nm wave [158]. The same authors showed that mouse embryos tolerate this method of warming well. Moreover, the viability of such frozen-thawed embryos was significantly higher compared to embryos thawed by the conventional convective method [158].
Khosla et al. later adapted this method for rapid heating of vitrified danio rerio embryos [22, 90, 144]. For fish embryos, a full replication of the technique developed for mouse embryos is unacceptable because the large size would firstly lead to too large temperature gradients during heating, and secondly, the internal structures of the embryo would still not be heated fast enough to prevent devitrification. Therefore, for experiments with danio rerio embryos as a heat receiver, instead of soot microparticles, the researchers used biocompatible pegylated plasmon resonance gold nanorods, which were placed not only in the external solution, but also injected into the yolk sac of the embryo.
Due to the use of ultrafast laser heating combined with microinjection of cryoprotectant and introduction of gold nanorods into the yolk, the first frozen-thawed embryos of danio rerio fish were obtained that not only preserved their structural integrity but also were able to develop normally after thawing. Nine percent of the freeze-thawed embryos developed to the hatching stage, and 2 out of 282 embryos cryopreserved using this method reached sexual maturity and successfully produced normal offspring [22]. However, despite the apparent success, the problem of cryopreservation of fish and amphibian embryos has not been solved yet: the described method is laborious, expensive and not very efficient. It is hardly suitable for mass application in aquaculture and fish farms. Nevertheless, the results obtained give hope for a successful solution of the problem of cryopreservation of oocytes and embryos of fish and amphibians in the future.
5.1.3. Prevention of low-temperature cracking
Cracking has not been practically investigated for oocytes and fish embryos, and, accordingly, methods of its prevention for these objects have not been developed. It is known from publications on vitrification of organs and tissues that the degree of low-temperature cracking can be reduced by sharply slowing the cooling rate near the vitrification temperature. In this case, the temperature gradients equalize and the degree of thermomechanical stress is reduced [146, 151]. For the same purpose, samples of structures such as blood vessels and cartilage fragments are not stored in liquid nitrogen but at temperatures on the order of -135°C. A smaller difference between the vitrification and storage temperatures also reduces the mechanical stress and prevents cracking [151, 161, 162].
Although cracking was not investigated in fish, Mixon et al. [163] made an interesting observation when experimenting the vitrification of finch (Misgurnus fosillis) embryos in a solution of 30% sucrose, 10% ethylene glycol, 20% 1,2-propanediol, and 3% PEG. Freezing was performed by plating the embryo with a drop of the solution on a pre-cooled copper plate. The authors noted that if the plate temperature was below -170°C, the drop of solution with the embryo was covered with small cracks and its structural integrity was broken; however, if the plate temperature was in the range of minus (109÷170)°C, the drop remained transparent after freezing and the embryo was visually intact. Unfortunately, the authors failed to obtain alive embryos after thawing [163].
6. Hypothermic storage of fish and amphibian oocytes and embryos
6.1. Hypothermic storage of fish oocytes and embryos
Due to the fundamental problems in the field of cryopreservation of oocytes and embryos of fish and amphibians, the researchers focus their attention to the possibility of improving the technologies of hypothermic storage.
The most interesting aspect is the prolongation of hypothermic storage of eggs and embryos of commercial fish. It should be noted that there are pronounced species differences in cold resistance of eggs and embryos of different fish species, which affect the results and time of storage of biomaterial at near-zero temperatures. For example, it has been shown that coho salmon eggs can be stored for up to four months at 1.4°C if previously incubated at 7.6°C for 13 days [164]. Harvey et al. [51] reported that unfertilized salmonid eggs can be stored at -1°C for up to 20 days in artificial media and ovarian fluid.
The survival rate of fish embryos during hypothermic storage is influenced not only by storage temperature; it also depends on the embryo development stage. For example, danio rerio embryos within the period of 27 to 40 hours after fertilization were the least sensitive to cooling, their survival rate made up 98.5±1.5% and 55.6±7.6% after 10 and 18 hours at 0°C, respectively [94]. In addition, the results of hypothermic storage of fish embryos are influenced by the presence of cryoprotectants in the storage medium. For example, Pullin and Bailey observed an increase in survival rates of flounder (Pleuronectes platessa) eggs during storage at near-zero temperatures when 1 M methanol was added to the storage medium. When this cryoprotective agent was added to the medium, survival rates of flounder eggs were 88.4±10.7%, 81.8±9.2%, and 30.2±3.5% after storage for 18, 24, and 48 hours at 0°C, respectively [165]. Some authors reported the dependence of the rate of fish embryos development on the oxygen level in the storage medium. For example, in studies on the effect of oxygen and temperature on the development of brook trout and rainbow trout embryos, it was shown that the rate of their development decreased due to lower levels of dissolved oxygen [166].
6.2. Hypothermic storage of amphibian oocytes and embryos
The application of currently known methods of cryopreservation of biological objects for deep freezing of oocytes and early amphibian embryos has not yet yielded positive results. This has contributed to the continuing interest in the study of the hypothermic storage of ovulated oocytes and early embryos at low positive temperatures [9].
In early studies, oocytes were stored in water and then in physiologic solutions, in particular, in Ringer solution for amphibians (SAR). It was shown that when oocytes were stored in solutions with low osmolality (5 mOsm kg-1), their fertilization capacity decreased to zero after 30-60 minutes [167]. When stored in SAR, ovulated oocytes of Rhinella (Bufo) marinus lost fertilization capacity after 8 hours [96], while Limnodynastes tasmaniensis oocytes lost fertilization capacity after 12-16 hours of storage [168]. In addition, a successful storage of oocytes of tropical amphibian species in SAR physiological solution was found to be dependent on storage temperature. For example, the optimal temperature for storage of Rhinella marinus oocytes was 15°C [96], and 10°C for Bufo fowleri oocytes[169]. Storage of R. marinus oocytes at 4°C invariably resulted in a rapid loss of fertilization capacity [96].
The reasons for the relatively rapid decline in oocyte fertilization capacity when oocytes are stored in various aqueous solutions, both of low and high osmolality, are not well understood yet [13, 169]. Perhaps one of the reasons is the saturation of the oocyte gelatinous envelope with water from storage solutions, which leads to its significant swelling and changes in the structure and properties. Therefore, in recent publications it was proposed to preserve ovulated oocytes “dry” in small, tightly closed bouquets without using any solutions [8,170]. In these bouquets, oocytes do not dry out, and in the absence of any solutions, the gelatinous envelope of oocytes does not swell. In these studies, it was shown that after 5 days of storage about 60% of Rana temporaria oocytes retained the ability to fertilize, and only by the ninth day of storage the percentage of oocytes capable of fertilization decreased to almost zero [8,170]. Thus, the proposed “dry” method of hypothermic storage of ovulated amphibian oocytes without any aqueous solutions increased the storage duration from 8-12 hours, to 6-7 days.
The “dry” method was further developed in studies of Gagarinskiy et al. [171]. For hypothermic storage of grass frog oocytes, a specialized chamber for preservation under the pressure of a gas mixture was used. Experimental oocytes stored in a gas mixture (O2 + CO) at 6.5 atm pressure showed high fertilization rates: 66%±8 % fertilization after 7, and 39±14 % after 12 days of storage. For the control groups of oocytes without gas, stored in closed bouquets in the refrigerator, fertilization rates were 41±12% after 7 days and 0% after 12 days of storage. Thus, the use of a modified “dry” method of hypothermic storage of oocytes in a pressurized atmosphere of gases (O2 + CO) helped to increase the duration of their storage to at least 12 days [171].
Of equal interest are the methods of hypothermic preservation of ovulated oocytes at reduced temperature (4°C) in the oviducts of decapitated or live females of the grass frog R. temporaria [8, 170]. In the first case, after hormonal stimulation and completion of ovulation of oocytes, experimental frogs were decapitated, and their carcasses with oocytes in oviducts, were placed in a refrigerator. It was found that even after 8 days of cold storage of oocytes in the carcasses of female grass frogs, about 40% of oocytes retained the ability to fertilize, and about 18% of them successfully developed to the hatching stage [8].
When oocytes were stored in live females as proposed by these authors, the time of oocyte viability preservation was significantly increased. After ovulation of oocytes, the grass frog females were kept in a refrigerator at 4°C, which is 3-5 degrees below the natural spawning temperature of this amphibian species. The result was the inhibition of their reproductive activity and prolongation of the preservation of ovulated oocytes in the oviducts. It was found that oocytes kept in alive females in a refrigerator for 30 days retained fertilization capacity in 46, 4 ± 3% of experiments, and 49.2 ± 8 ± 8% of these fertilized oocytes successfully developed to hatching [8, 170]. In a later study, these authors showed that even after 70 days of keeping grass frog females with ovulated oocytes in a refrigerator, about 75.8 ± 8.1% of the retrieved oocytes were successfully fertilized and 74.8 ± 8.4% of these oocytes developed to hatching [172].
Thus, the analysis of the literature indicates that during refrigerated storage of ovulated oocytes in various solutions, their ability to fertilization is limited to hours or tens of hours. When oocytes are stored in carcasses of female amphibians, the period of their successful preservation increases to 9-10 days. The duration of cold storage of oocytes by the “dry” method in tightly closed bouquets also does not exceed 9-10 days, and the improved “dry” method using pressurized gas mixtures in chambers for gas preservation increases this period up to 12 days. The record duration was observed for hypothermic storage of grass frog oocytes in the bodies of living females. In the published study it was 2-2.5 months.
It is known that the rate of development of amphibian embryos depends on the temperature of their environment. By example of larvae of Rana temporaria frog, it was shown that the duration of one mitotic cycle during the division period, and, consequently, the rate of larval development, was proportional to the water temperature in which the larvae were kept. The lower the water temperature, the longer the mitotic cycle and the slower the rate of development of the embryos [81]. In addition, it was found that at 4°C in a refrigerator the extremely slowly developing R. temporaria embryos were unable to reach the hatching stage (Uteshev, unpublished data). Similar results were reported by Ruthsatz et al. who showed that R. temporaria larvae stopped developing and failed to undergo metamorphosis at water temperature of 10°C [173].
A significant positive correlation was also shown between the body length of larvae of the rough-skinned newt Taricha granulosa (western North America) and the holding temperature of these animals [174]. A similar pattern of dependence of developmental rate on water temperature during housing was found in tadpoles of the wood frog Lithobates sylvaticus from North America. Research indicated a direct inverse relationship between water temperature during housing and both the developmental rate and weight gain of tadpoles [175].
Conclusion
The primary obstacles to successful cryopreservation of mature oocytes and embryos in fish and amphibians include low permeability of their multilayered envelopes, substantial yolk volume, elevated intracellular ice nucleation temperature, and large size. Low permeability impedes cryoprotectant penetration, the significant yolk mass adversely affects chilling tolerance, and the high ice nucleation temperature renders slow freezing ineffective. The considerable size restricts cooling/warming rates during vitrification and may induce low-temperature fracturing.
Various approaches—including ultrasound application, aquaporin mRNA introduction, laser treatment, hydrostatic pressure, electroporation, osmotic and chemical treatments, and cryoprotectant microinjection— are being investigated to enhance envelope permeability and chilling tolerance. Vitrification shows promise but necessitates ultra-rapid warming, presenting significant technical challenges for large specimens. Partial yolk removal has yielded positive outcomes in select species.
Hypothermic storage of fish and amphibian oocytes and embryos at low positive temperatures is also actively explored as a cryopreservation alternative. Current methods extend storage duration to days or weeks, though scalability remains limited.
Despite advances such as combined microinjection and laser warming, comprehensive cryopreservation of fish and amphibian oocytes/embryos remains unrealized due to methodological complexity, high costs, and suboptimal efficiency. Nevertheless, achieved milestones provide grounds for cautious optimism in future developments.
References
- IUCN Red List of Threatened Species. Доступен по ссылке: https://www.iucnredlist.org/resources/summary-statistics (дата посещения 27.06.2024).
- González-Del-Pliego P., Freckleton R. P., Edwards D. P., Koo M. S., Scheffers B. R., Pyron R. A., and Jetz W. Phylogenetic and Trait-Based Prediction of Extinction Risk for Data-Deficient Amphibians. Curr. Biol., 29 (9), 1557-1563.e3 (2019). DOI: 10.1016/j.cub.2019.04.005.
- Scheele B. C., Pasmans F., Skerratt L. F., Berger L., Martel A., Beukema W., Acevedo A. A., Burrowes P. A., Carvalho T., Catenazzi A., De la Riva I., Fisher M. C., Flechas S. V., Foster C. N., Frías-Álvarez P., Garner T. W. J., Gratwicke B., Guayasamin J. M., Hirschfeld M., Kolby J. E., Kosch T. A., La Marca E., Lindenmayer D. B., Lips K. R., Longo A. V., Maneyro R., McDonald C. A., Mendelson J. 3rd, Palacios-Rodriguez P., Parra-Olea G., Richards-Zawacki C. L., Rödel M. O., Rovito S. M., Soto-Azat C., Toledo L. F., Voyles J., Weldon C., Whitfield S. M., Wilkinson M., Zamudio K. R., and Canessa S. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science, 363 (6434), 1459-1463 (2019). DOI: 10.1126/science.aav0379.
- Sumaila U. R., and Tai T. C. End Overfishing and Increase the Resilience of the Ocean to Climate Change. Front. Marine Sci., 7, 523 (2020). DOI: 10.3389/fmars.2020.00523.
- Miranda R., Miqueleiz I., Darwall W., Baker J., Ensikat K., GofF G., Hamman E., Irvine K., Juffe-Bignoli D., Langhammer P.F., Martin R., Pacifici M., Smith K., Polidoro B., Soulsby A.-M., Steininger M., Vie J.-C., and de Grammont P. C. Monitoring extinction risk and threats of the world’s fishes based on the Sampled Red List Index. Rev. Fish Biol. Fisheries, 32, 975–991 (2022). DOI: 10.1007/s11160-022-09710-1.
- Tsai S., and Lin C. Advantages and Applications of Cryopreservation in Fisheries Science. Braz. Arch. Biol. Technol., 55 (3), 425-433 (2012). DOI: 10.1590/s1516-89132012000300014.
- Torres L., Hu E., and Tiersch T. R. Cryopreservation in fish: current status and pathways to quality assurance and quality control in repository development. Reprod. Fertil. Dev., 7 (10), 1071 (2016). DOI: 10.1071/RD15388.
- Uteshev V. K., Gakhova E. N., Kramarova L. I., Shishova N. V., Kaurova S. A., and Browne R. K. Cryobanking of amphibian genetic recourses in Russia: past and future. Russ. J. Herpetol., 26, 319-324 (2019). DOI: 10.30906/1026-2296-2019-26-6-319-324.
- Anastas Z. M., Byrne P. G., O’Brien J. K., Hobbs R. J., Upton R., and Silla A. J. The Increasing Role of Short-Term Sperm Storage and Cryopreservation in Conserving Threatened Amphibian Species. Animals, 13 (13), 2094 (2023). DOI: 10.3390/ani13132094.
- Browne R. K., Silla A. J., Upton R., Della-Togna G., Marcec-Greaves R., Shishova N. V., Uteshev V. K., Proaño B., Pérez O. D., Mansour N., Kaurova S. A., Gakhova E. N., Cosson J., Dyzuba B., Kramarova L. I., McGinnity D., Gonzalez M., Clulow J., and Clulow S. Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology, 133, 187-200 (2019).
- Browne R. K., Luo Q., Wang P., Mansour N., Kaurova S. A., Gakhova E. N., Shishova N. V., Uteshev V. K., Kramarova L. I., Venu G., Ma L., Qin B., Li W., Wu B., Liu X., Yang C., Li Y., Liu B., Zhu Q., Hou Y., Ren J., and Rawson D. M. The Sixth Mass Extinction and Amphibian Species Sustainability Through Reproduction and Advanced Biotechnologies, Biobanking of Germplasm and Somatic Cells, and Conservation Breeding Programs (RBCs). Animals, 14 (23), 3395 (2024). DOI: 10.3390/ani14233395.
- Clulow J., and Clulow S. Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the arts up to speed. Reprod. Fertil. Dev., 28, 1116–1132 (2016). DOI: 10.1071/RD15466.
- Clulow J., Upton R., Trudeau V. L., and Clulow S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. In Reproductive Sciences in Animal Conservation, Ed. by Comizzoli P., Brown J., and Holt W. (Cham, Springer, 2019), vol. 1200, pp. 295–319. DOI: 10.1007/978-3-030-23633-5_14.
- Kopeyka E.F., Petrushko M.P., Pinyaev V.I., Yurchuk T.A., Pavlovich E.V., Mikson K.B., Butsky K.I., Gapon A.A., and Pugovkin A.Yu. Cryopreservation of reproductive cells and embryos of laboratory, farm, and wild animals. Cryobiol. Cryomed., 29 (1), 3–18 (2019). DOI:10.15407/cryo29.01.003.
- Diwan A. D., Harke S. N., Gopalkrishna, and Panche А. N. Cryobanking of Fish and Shellfish Egg, Embryos and Larvae: An Overview. Front. Mar. Sci., 7, 251 (2020). DOI: 10.3389/fmars.2020.00251.
- Martínez-Páramo S., Horváth Á., Labbé C., Zhang T., Robles V., Herráez P., Suquet M., Adams S., Viveiros A., Tiersch T. R., and Cabrita E. Cryobanking of aquatic species. Aquaculture, 472, 156-177 (2017). DOI: 10.1016/j.aquaculture.2016.05.042.
- Chen S. L., and Tian Y. S. Cryopreservation of flounder (Paralichthys olivaceus) embryos by vitrification. Theriogenology, 63 (4), 1207-1219 (2005). DOI: 10.1016/j.theriogenology.2004.06.007
- Tsai S., Rawson D. M., and Zhang T. Development of cryopreservation protocols for early stage zebra fish (Danio rerio) ovarian follicles using controlled slow cooling. Theriogenology, 71, 1226–1233 (2009). DOI: 10.1016/j.theriogenology.2009.01.014.
- Plachynta M. Studies on cryopreservation of zebrafish (Danio Rerio) oocytes using controlled slow cooling. (2007). Доступен по ссылке: http://hdl.handle.net/10547/622016.
- Keivanloo S., and Sudagar M. Cryopreservation of Persian sturgeon (Acipenser persicus) embryos by DMSO-based vitrificant solutions. Theriogenology, 85 (5), 1013-1018 (2016). DOI: 10.1016/j.theriogenology.2015.11.012.
- Zhang J., Tian Y., Li Z., Wu Y., Li Z., Cheng M., Wang L., Ma W., and Zhai J. Optimization of vitrification factors for embryo cryopreservation of kelp grouper (Epinephelus moara). Theriogenology, 142, 390-399 (2020). DOI: 10.1016/j.theriogenology.2019.10.002.
- Khosla K., Kangas J., Liu Y., Zhan L., Daly J., Hagedorn M., and Bischof J. Cryopreservation and Laser Nanowarming of Zebrafish Embryos Followed by Hatching and Spawning. Adv. Biosyst., 4 (11), e2000138 (2020). DOI: 10.1002/adbi.202000138.
- Zhang M., Ma X., Han Y., Wang Z., Jia Z., Chen D., Qiao Z., Gao X., Zhao C., Shen Y., Optimal conditions for cryopreservation by vitrification of largemouth bass (Micropterus salmoides) embryos, Anim. Reprod. Sci., 270, 107613 (2024). DOI: h10.1016/j.anireprosci.2024.107613.
- Frisbie M. P., Costanzo J. P., and Lee R. E. Jr. Physiological and ecological aspects of low temperature tolerance in embryos of the wood frog, Rana sylvatica. Canad. J. Zool., 78, 1032-1041 (2000). DOI: 10.1139/z00-022.
- Derakhshan Z., Nokhbatolfoghahai M., and Zahiri S. Cryopreservation of Bufotes viridis embryos by vitrification. Cryobiology, 75, 60-67 (2017). DOI: 10.1016/j.cryobiol.2017.02.003.
- Kleinhans F. W., Guenther J. F., Roberts D. M., and Mazur P. Analysis of intracellular ice nucleation in Xenopus oocytes by differential scanning calorimetry. Cryobiol., 52, 128-138 (2006). DOI: 10.1016/j.cryobiol.2005.10.008.
- Rall W. F. Recent advances in the cryopreservation of salmonids fishes. In Genetic conservation of Salmonids fishes, Ed. by Cloud J. G. and Thorgard G. H. (New York, Plenum Press, 1993), pp. 138–158.
- Hagedorn M., Peterson A., Mazur P., and Kleinhans F. W. High ice nucleation temperature of zebrafish embryos: slow freezing is not an option. Cryobiology, 49 (2), 181–189 (2004). DOI: 10.1016/j.cryobiol.2004.07.001.
- Asturiano J. F., Cabrita E., and Ákos H. Progress, challenges and perspectives on fish gamete cryopreservation: a mini review general and comparative. Endocrinology, 45, 69–76 (2017). DOI: 10.1016/j.ygcen.2016.06.019.
- Barisone G. A., Albertali I. E., Sánchez M., and Cabada M. O. The envelopes of amphibian oocytes: physiological modifications in Bufo arenarum. Reprod. Biol. Endocrinol., 1, 18 (2003). DOI: 10.1186/1477-7827-1-18.
- Arukwe A., and Goksøyr A. Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp. Hepatol., 2 (1), 4 (2003). DOI: 10.1186/1476-5926-2-4.
- Rizzo E., and Bazzoli N. Reproduction and embryogenesis. In Biology and Physiology of Freshwater Neotropical Fish (Elsevier, 2020), pp. 287-313. DOI: 10.1016/B978-0-12-815872-2.00013-0.
- Valdebenito I., Figueroa E., Valdebenito M., and Paiva L. Chorion Alterations in Eyed-Stage Salmonid Eggs Farmed in La Araucanía, Chile: A Retrospective Study. Animals (Basel), 11 (8), 2427 (2021). DOI: 10.3390/ani11082427.
- Pérez-Atehortúa M., Hernández A. J., Dantagnan P., Silva M., Risopatrón J., Farías J., Villalobos E. F., and Valdebenito I. Chorion in fish: Synthesis, functions and factors associated with its malformations. Aquac. Rep., 30, 101590 (2023). DOI: 10.1016/j.aqrep.2023.101590.
- Kalayda M.L. General Histology and Embryology of Fish. Practical Course: Textbook (Prospekt Nauki, Saint Petersburg, 2024), 88 p. (URL: http://m.ibooks.ru/bookshelf/353761/reading, accessed: 23.10.2024. ISBN: pn_0037.)
- Altig R., and McDiarmid R. W. Morphological diversity and evolution of egg and clutch structure in amphibians. Herpetol. Monogr., 21 (1), 1–32 (2007). DOI: 10.1655/06-005.1.
- Siddique M. A. M., Cosson J., Psenicka M., and Linhart O. A review of the structure of sturgeon egg membranes and of the associated terminology. J. Appl. Ichthyol., 30 (6), 1246-1255 (2014). DOI: 10.1111/jai.12604.
- Litscher E. S., and Wassarman P. M. Evolution, structure, and synthesis of vertebrate egg-coat proteins. Trends Dev. Biol., 8, 65-76 (2014). PMID: 26504367; PMCID: PMC4618670.
- Litscher E. S., and Wassarman P. M. (Eds.). Extracellular Matrix and Egg Coats. (2018), Vol. 130. (Hardback ISBN: 9780128098028; eBook ISBN: 9780128098035).
- Fliniaux I., Marchand G., Molinaro C., Decloquement M., Martoriati A., Marin M., Bodart J. F., Harduin-Lepers A., and Cailliau K. Diversity of sialic acids and sialoglycoproteins in gametes and at fertilization. Front. Cell Dev. Biol., 10, 982931 (2022). DOI: 10.3389/fcell.2022.982931.
- Böhme S., Baccaro M., Schmidt M., Potthoff A., Stärk H-J., Reemtsma T., and Kühnel D. Metal uptake and distribution in the zebrafish (Danio rerio) embryo: differences between nanoparticles and metal ions. Environ. Sci.: Nano, 4, 1005-1015 (2017). DOI: 10.1039/C6EN00440G.
- Cotelli F., Andronico F., Brivio M., and Lamia C. L. Structure and composition of the fish egg chorion (Carassius auratus). J. Ultrastruct. Mol. Struct. Res., 99 (1), 70-78 (1988). DOI: 10.1016/0889-1605(88)90034-1.
- Nakaya K., White W. T., and Ho H. C. Discovery of a new mode of oviparous reproduction in sharks and its evolutionary implications. Sci. Rep., 10 (1), 12280 (2020). DOI: 10.1038/s41598-020-68923-1.
- Cherr G. N., and Clark W. H., Jr. Fine Structure of the Envelope and Micropyles in the Eggs of the White Sturgeon, Acipenser transmontanus Richardson. Dev. Growth Differ., 24 (4), 341 - 352 (1982). DOI: 10.1111/j.1440-169X.1982.00341.x.
- Kurdyaeva V.P., Shkarina T.V., and Shapovalov M.E. On the formation and structure of egg envelopes in two species of gudgeons from Lake Khanka: Sarcoheilichthys czerskii (Berg) and Sarcoheilichthys sinensis (Bleeker). Izvestiya TINRO, (2003). URL: https://cyberleninka.ru/article/n/o-formirovanii-i-stroenii-yaytsevyh-obolochek-dvuh-vidov-peskarey-oz-hanka-sarcoheilichthys-czerskii-berg-i-sarcoheilichthys-sinensis (accessed: 18.10.2024).
- Carroll E. J. Jr., Wei S. H., Nagel G. M., and Ruibal R. Structure and Macromolecular of the Egg and Embryo Jelly Anuran Lepidobatrachus laevis. Develop. Growth Differ., 33 (l), 37-43 (1991).
- Bonnell B. S., and Chandler D. E. Egg jelly layers of Xenopus laevis are unique in ultrastructure and sugar distribution. Mol. Reprod. Dev., 44 (2), 212-20 (1996). DOI: 10.1002/(SICI)1098-2795(199606)44:2<212::AID-MRD10>3.0.CO;2-4.
- Hagedorn M., Lance S. L., Fonseca D. M., Kleinhans F. W., Artimov D., Fleischer R., Hoque A. T. M. S., Hamilton M. B., and Pukazhenthi B. S. Altering Fish Embryos with Aquaporin-3: An Essential Step Toward Successful Cryopreservation. Biol. Reprod., 67 (3), 961–966 (2002). DOI: 10.1095/biolreprod.101.002915.
- Zhang T., Isayeva A., Adams S. L., and Rawson D. M. Studies on membrane permeability of zebrafish (Danio rerio) oocytes in the presence of different cryoprotectants. Cryobiology, 50 (3), 285-93 (2005). DOI: 10.1016/j.cryobiol.2005.02.007.
- Wang R. Y., Zhang T., Bao Q., and Rawson D. M. Study on fish embryo responses to the treatment of cryoprotective chemicals using impedance spectroscopy. Eur. Biophys. J., 35 (3), 224-30 (2006). DOI: 10.1007/s00249-005-0027-5.
- Harvey B., Kelley R. N., and Ashwood-Smith M. J. Permeability of intact and dechorionated zebra fish embryos to glycerol and dimethyl sulfoxide. Cryobiology, 20 (4), 432-439 (1983). DOI: 10.1016/0011-2240(83)90033-0 .
- Hagedorn M., Hsu E. W., Pilatus U., Wildt D. E., Rall W. F., and Blackband S. J. Magnetic resonance microscopy and spectroscopy reveal kinetics of cryoprotectant permeation in a multicompartmental biological system. Proc. Natl. Acad. Sci. U.S.A., 93 (15), 7454-7459 (1996). DOI: 10.1073/pnas.93.15.7454.
- Hagedorn M., Hsu E., Kleinhans F. W., and Wildt D. E. New approaches for studying the permeability of fish embryos: toward successful cryopreservation. Cryobiology, 34 (4), 335-47 (1997a). DOI: 10.1006/cryo.1997.2014.
- Hagedorn M., Kleinhans F. W., Wildt D. E., and Rall W. F. Chill sensitivity and cryoprotectant permeability of dechorionated zebrafish embryos, Brachydanio rerio. Cryobiology, 34 (3), 251-63 (1997b). DOI: 10.1006/cryo.1997.2002.
- Hagedorn M., Kleinhans F. W., Freitas R., Liu J., Hsu E. W., Wildt D. E., and Rall W. F. Water distribution and permeability of zebrafish embryos, Brachydanio rerio. J. Exp. Zool., 278 (6), 356-71 (1997c). DOI: 10.1002/(sici)1097-010x(19970815)278:6<356::aid-jez3>3.0.co;2-n.
- Zhang R. B., and Werkman A. S. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am. J. Physiol. Cell Physiol., 260, C26–34 (1991). DOI: 10.1152/ajpcell.1991.260.1.C26.
- Lau Y. T., Reynhout J. K., and Horowitz S. B. Membrane permeability changes during Rana oocyte maturation. Experientia, 50, 606–609 (1994). DOI: 10.1007/BF01921732.
- Kleinhans F. W., Guenther J. F., Seki S., Edashige K., Roberts D. M., and Mazur P. Water Pf of Xenopus (AQP +/-) Stage I and II oocytes. Cryobiology, 51, 389 (2005).
- Chauvigné F., Zapater C., and Cerdà J. Role of Aquaporins during Teleost Gametogenesis and Early Embryogenesis. Front. Physiol., 2, 66 (2011). DOI: 10.3389/fphys.2011.00066.
- Wang R. Y., Guan M., Rawson D. M., and Zhang T. Ultrasound enhanced methanol penetration of zebrafish (Danio rerio) embryos measured by permittivity changes using impedance spectroscopy. Eur. Biophys. J., 37 (6), 1039-44 (2008). DOI: 10.1007/s00249-007-0229-0.
- Silakes S., and Bart A. N. Ultrasound enhanced permeation of methanol into zebrafish, Danio rerio, embryos. Aquaculture, 303 (1-4), 71–76 (2010). DOI: 10.1016/j.aquaculture.2010.02.018.
- Rahman S. M., Strüssmann C. A., Suzuki T., Majhi S. K., Hattori R. S., and Alam M. A. Effects of ultrasound on permeation of cryoprotectants into Japanese whiting Sillago japonica embryos. Cryobiology, 77, 19-24 (2017). DOI: 10.1016/j.cryobiol.2017.06.003.
- Mel’nikova E. V., Uteshev V. K., Pashovkin T. N., Sokolova A. A., Yashin V. A., Karnaukhov V. N., and Gakhova E. N. Changes in the permeability of amphibian embryo envelope for fluorochromes under the action of ultrasound. Biophysics, 50 (3), 500-4 (2005).
- Gakhova E.N., Pashovkin T.N., Melnikova E.V., and Uteshev V.K. A new approach to cryopreservation of early aquatic embryos: alteration of membrane permeability by ultrasound. In Biophysics of the Living Cell (Publisher and location not specified, 2006), Vol. 8, pp. 167–171.
- Gakhova E.N., Pashovkin T.N., Melnikova E.V., Uteshev V.K., and Sadikova D.G. Ultrasound can alter the permeability of amphibian embryo membranes. Veterinary Pathology, 23 (4), 238–240 (2007).
- Uteshev V.K., Pashovkin T.N., Sevirov A.N., Melnikova E.V., Sadikova D.G., Karnaukhov V.N., and Gakhova E.N. Survival of amphibian embryos after exposure to continuous ultrasound. Biophysics, 51 (3), 539–544 (2006).
- Uteshev V.K., Pashovkin T.N., and Gakhova E.N. Survival of amphibian embryos after exposure to modulated ultrasound in the therapeutic intensity range. Herald of New Medical Technologies, XVII (4), 170–172 (2010).
- Yamaji Y., Valdez D. M. Jr., Seki S., Yazawa K., Urakawa C., Jin B., Kasai M., Kleinhans F. W., and Edashige K. Cryoprotectant permeability of aquaporin-3 expressed in Xenopus oocytes. Cryobiology, 53 (2), 258-267 (2006). DOI: 10.1016/j.cryobiol.2006.06.008.
- Kohli V., Robles V., Cancela M. L., Acker J. P., Waskiewicz A. J., and Elezzabi A. Y. An alternative method for delivering exogenous material into developing zebrafish embryos. Biotechnol. Bioeng., 98 (6), 1230-1241 (2007). DOI: 10.1002/bit.21564.
- Routray P., Suzuki T., and Strüssmann C. A. Factors affecting the uptake of DMSO by the eggs and embryos of medaka, Oryzias latipes. Theriogenology, 58, 1483–1496 (2002). DOI: 10.1016/s0093-691x(02)01076-2.
- Rahman S. M., Strüssmann C. A., Suzuki T., and Watanabe M. Electroporation enhances permeation of cryoprotectant (dimethyl sulfoxide) into Japanese whiting (Sillago japonica) embryos. Theriogenology, 79 (5), 853-858 (2013). DOI: 10.1016/j.theriogenology.2013.01.002.
- Rahman S. M., Strüssmann C. A., Majhi S. K., Suzuki T., and Watanabe M. Efficiency of osmotic and chemical treatments to improve the permeation of the cryoprotectant dimethyl sulfoxide to Japanese whiting (Sillago japonica) embryos. Theriogenology, 75 (2), 248-255 (2011). DOI: 10.1016/j.theriogenology.2010.08.011.
- Leung L. K. B., and Jamieson B. G. M. Live preservation of fish gametes. In Fish Evolution and Systematic: Evidence from Spermatozoa, Ed. by Jamieson B. G. M. (Cambridge, MA, Cambridge University Press, 1991), pp. 245–269.
- Beirão J., Robles V., Herráez M. P., Sarasquete C., Dinis M. T., and Cabrita E. Cryoprotectant microinjection toxicity and chilling sensitivity in gilthead seabream (Sparus aurata) embryos. Aquaculture, 261 (3), 897-903 (2006). DOI: 10.1016/j.aquaculture.2006.07.039.
- Alam M. A., Rahman S. M., Yamamoto Y., Hattori R. S., Suzuki T., Watanabe M., and Strüssmann C. A. Optimization of protocols for microinjection-based delivery of cryoprotective agents into Japanese whiting Sillago japonica embryos. Cryobiology, 85, 25-32 (2018). DOI: 10.1016/j.cryobiol.2018.10.007.
- Jevtić P., Elliott K. W., Watkins S. E., Sreter J. A., Jovic K., Lehner I. B., Baures P. W., Tsavalas J. G., Levy D. L., and Varga K. An insect antifreeze protein from Anatolica polita enhances the cryoprotection of Xenopus laevis eggs and embryos. J. Exp. Biol., 225 (4), jeb243662 (2022). DOI: 10.1242/jeb.243662.
- Wallace R. A. Vitellogenesis and oocyte growth in nonmammalian vertebrates. In Oogenesis, Ed. by Browder L. W. (Boston, Springer, 1985), pp. 127–77.
- Heming T., and Buddington R. Yolk Absorption in Embryonic and Larval Fishes. Fish Physiology, 11, 408–438 (1988). DOI: 10.1016/S1546-5098(08)60203-4.
- Wallace R. A., and Karasaki S. Studies on amphibian yolk 2. The isolation of yolk platelets from the eggs of Rana pipiens. J. Cell Biol., 18 (1), 153-166 (1963). DOI: 10.1083/jcb.18.1.153.
- Makeeva A.P. Embryology of Fish (MSU Publishing House, Moscow, 1992), 216 p. ISBN: 5-211-01647-5.
- Dabagyan N.V. and Sleptsova L.A. The common frog Rana temporaria In Developmental Biology Objects, ed. by Astaurov E.A. and Detlaf T.A. (Nauka, Moscow, 1975), pp. 422–462.
- Wallace R. A. Studies on amphibian yolk III. A resolution of yolk platelet components. Biochim. Biophys. Acta, 74, 494–504 (1963). DOI: 10.1016/0006-3002(63)91392-1.
- Kondakova E. A., Efremov V. I. i Kozin V. V. Obshie i specificheskie cherty organizacii zheltochnogo sincitial'nogo sloya teleostei na primere Gasterosteus aculeatus. Izvestiya RAN. Seriya biologicheskaya, 1, 31–37 (2019a). DOI: 10.1134/S0002332919010028. Article in Russian.
- Kondakova E. A., Shkil' F. N. i Efremov V. I. Struktura zheltochnogo sincitial'nogo sloya v postembrional'nom razvitii biryuzovoi akary, Andinoacara rivulatus (Günther), 1860 (Cichlidae). Trudy Zoologicheskogo instituta RAN, 323 (4), 523-532 (2019b). Article in Russian.
- Fuentes R., and Fernández J. Ooplasmic segrega-tion in the zebrafish zygote and early embryo: pattern of ooplasmic movements and transport pathways. Dev. Dyn., 239 (8), 2172–2189 (2010). DOI: 10.1002/dvdy.22349.
- Yamamoto K. Periblast in the egg of the eel, Anguilla japonica. Jpn. J. Ichthyol., 28, 423-430 (1982).
- Chu L. T., Fong S. H., Kondrychyn I., Loh S. L., Ye Z., and Korzh V. Yolk syncytial layer formation is a failure of cytokinesis mediated by Rock1 function in the early zebrafish embryo. Biol. Open, 1 (8), 747–753 (2012). DOI: 10.1242/bio.20121636.
- Hagedorn M., Kleinhans F. W., Artemov D., and Pilatus U. Characterization of a Major Permeability Barrier in the Zebrafish Embryo. Biol. Reprod., 59 (5), 1240–1250 (1998). DOI: 10.1095/biolreprod59.5.1240.
- Liu X-H., Zhang T., and Rawson D. M. Differential scanning calorimetry studies of intramembrane freezing and cryoprotectant penetration in zebrafish (Danio rerio) embryos. J. Exp. Zool., 290, 299–310 (2001a). DOI: 10.1002/jez.1060.
- Khosla K., Wang Y., Hagedorm M., Qin Z., and Bischof J. Gold nanorod induced warming of embryos from the cryogenic state enhances viability. ASC Nano, 11, 7869–7878 (2017). DOI: 10.1021/acsnano.7b02216.
- Dinnyés A., Urbányi B., Baranyai B., and Magyary I. Chilling sensitivity of carp (Cyprinus carpio) embryos at different developmental stages in the presence or absence of cryoprotectants: work in progress. Theriogenology, 50 (1), 1-13 (1998). DOI: 10.1016/s0093-691x(98)00108-3.
- Liu X. H., Zhang T., and Rawson D. M. The effect of partial removal of yolk on the chilling sensitivity of zebrafish (Danio rerio) embryos. Cryobiology, 39 (3), 236-42 (1999). DOI: 10.1006/cryo.1999.2206.
- Valdez D. M. Jr., Miyamoto A., Hara T., Edashige K., and Kasai M. Sensitivity to chilling of medaka (Oryzias latipes) embryos at various developmental stages. Theriogenology, 64 (1), 112-22 (2005). DOI: 10.1016/j.theriogenology.2004.11.006.
- Zhang T. T., and Rawson D. M. Studies on chilling sensitivity of zebrafish (Brachydanio rerio) embryos. Cryobiology, 32, 239-246 (1995). DOI: 10.1016/j.cryobiol.2004.05.005.
- Zhang T., Liu X.-H., and Rawson D. M. Effects of methanol and developmental arrest on chilling injury in zebra fish (Danio rerio) embryos. Theriogenology, 59, 1545–1556 (2003). DOI: 10.1016/s0093-691x(02)01199-8.
- Browne R. K., Clulow J., and Mahony M. Short-term storage of cane toad (Bufo marinus) gametes. Reproduction, 121 (1), 167–173 (2001). DOI: 10.1530/rep.0.1210167.
- Kouba A. J., Lloyd R. E., Houck M. L., Silla A. J., Calatayud N., Trudeau V. L., Comizzoli P., and Germano J. M. Emerging trends for biobanking amphibian genetic resources: the hope, reality and challenges for the next decade. Biol. Conserv., 164, 10–21 (2013). DOI: 10.1016/j.biocon.2013.03.010.
- Lawson B., Clulow S., Mahony M. J., and Clulow J. Towards gene banking amphibian maternal germ lines: short-term incubation, cryoprotectant tolerance and cryopreservation of embryonic cells of the frog, Limnodynastes peronii. PLoS One, 8 (4), e60760 (2013). DOI: 10.1371/journal.pone.0060760.
- Clulow J., Trudeau V. L., and Kouba A. J. Amphibian declines in the twenty-first century: why we need assisted reproductive technologies. In Reproductive sciences in animal conservation, Advances in experimental medicine and biology 753, Ed. by Holt W. V., et al. (New York, Springer, 2014), pp. 275–316. DOI: 10.1007/978-1-4939-0820-2_12.
- Liu X. H., Zhang T., and Rawson D. M. Effect of cooling rate and partial removal of yolk on the chilling injury in zebrafish (Danio rerio) embryos. Theriogenology, 55 (8), 1719-31 (2001b). DOI: 10.1016/s0093-691x(01)00515-5.
- Higaki S., Eto Y., Kawakami Y., Yamaha E., Kagawa N., Kuwayama M., Nagano M., Katagiri S., and Takahashi Y. Production of fertile zebrafish (Danio rerio) possessing germ cells (gametes) originated from primordial germ cells recovered from vitrified embryos. Reproduction, 139 (4), 733-40 (2010). DOI: 10.1530/REP-09-0549.
- Higaki S., Kawakami Y., Eto Y., Yamaha E., Nagano M., Katagiri S., Takada T., and Takahashi Y. Cryopreservation of zebrafish (Danio rerio) primordial germ cells by vitrification of yolk-intact and yolk-depleted embryos using various cryoprotectant solutions. Cryobiology, 67 (3), 374-82 (2013). DOI: 10.1016/j.cryobiol.2013.10.006.
- Amstislavsky S.Ya., Mokrousova V.I., Okotrub S.V., Brusentsev E.Yu., and Naprimerov V.A. Application of the cryobank concept to wild and endangered species of the order Carnivora. Ontogenesis, 52 (5), 345–366 (2021). DOI: 10.31857/S0475145021040029.
- Amstislavsky S., Mokrousova V., Brusentsev E., Okotrub K., and Comizzoli P. Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos: a review. Biopreserv. Biobank., 17 (1), 76–83 (2019). DOI: 10.1089/bio.2018.0039.
- Spate L. D., Murphy C. N., and Prather R. S. High-throughput cryopreservation of in vivo-derived swine embryos. PLoS One, 8 (6), e65545 (2013). DOI: 10.1371/journal.pone.0065545.
- Sun X., Li Z., Yi Y., Chen J., Leno G. H., and Engelhardt J. F. Efficient term development of vitrified ferret embryos using a novel pipette chamber technique. Biol. Reprod., 79 (5), 832-40 (2008). DOI: 10.1095/biolreprod.107.067371.
- Abe H., Yamashita S., Satoh T., and Hoshi H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev., 61, 57–66 (2002). DOI: 10.1002/mrd.1131.
- Mokrousova V. I., Okotrub K. A., Brusentsev E. Y., Kizilova E. A., Surovtsev N. V., and Amstislavsky S. Y. Effects of slow freezing and vitrification on embryo development in domestic cat. Reprod. Domest. Anim., 55 (10), 1328-1336 (2020). DOI: 10.1111/rda.13776.
- Okotrub K. A., Mokrousova V. I., Amstislavsky S. Y., and Surovtsev N. V. Lipid droplet phase transition in freezing cat embryos and oocytes probed by Raman spectroscopy. Biophys. J., 115 (3), 577–587 (2018). DOI: 10.1016/j.bpj.2018.06.019.
- Quinn P. J. A lipid-phase separation model of low temperature damage to biological membranes. Cryobiology, 22, 128–146 (1985). DOI: 10.1016/0011-2240(85)90167-1.
- Drobnis E. Z., Crowe L. M., Berger T., Anchordoguy T. J., Overstreet J. W., and Crowe J. H. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J. Exp. Zool., 265 (4), 432-7 (1993). DOI: 10.1002/jez.1402650413.
- Wang R., Ma Y., Zhang L., Zhang Z., Chi Y., and Chi Y. Changes in egg yolk gelation behaviour and mechanisms during freezing. LWT - Food Sci. Technol., 151, 112223 (2021). DOI: 10.1016/j.lwt.2021.112223.
- El-Battawy K. A., and Linhart O. Preliminary studies on cryopreservation of common tench (Tinca tinca) embryos (work in progress). Reprod. Domest. Anim., 44 (4), 718-23 (2009). DOI: 10.1111/j.1439-0531.2007.01059.x.
- Tian Y., Chen Z., Tang J., Duan H., Zhai J., Li B., Ma W., Liu J., Hou Y., and Sun Z. Effects of cryopreservation at various temperatures on the survival of kelp grouper (Epinephelus moara) embryos from fertilization with cryopreserved sperm. Cryobiology, 75, 37-44 (2017). DOI: 10.1016/j.cryobiol.2017.02.007.
- Lin C., Kuo F.-W., Chavanich S., and Viyakarn V. Membrane lipid phase transition behavior of oocytes from three gorgonian corals in relation to chilling injury. PLoS One, 9 (3), e92812 (2014). DOI: 10.1371/journal.pone.0092812.
- Pearl M., and Arav A. Chilling sensitivity in zebrafish (Brachydanio rerio) oocytes is related to lipid phase transition. Cryo Letters, 21 (3), 171-178 (2000).
- Trinkaus J. P., and Drake J. W. Exogenous control of morphogenesis in isolated Fundulus blastoderms by nutrient chemical factors. J. Exp. Zool., 132, 311-47 (1956).
- Janik M., Kleinhans F. W., and Hagedorn M. Overcoming a permeability barrier by microinjecting cryoprotectants into zebrafish embryos (Brachydanio rerio). Cryobiology, 41 (1), 25-34 (2000). DOI: 10.1006/cryo.2000.2261.
- Batterman S. A., and Franzblau A. Time-resolved cutaneous absorption and permeation rates of methanol in human volunteers. Int. Arch. Occup. Environ. Health, 70 (5), 341-51 (1997). DOI: 10.1007/s004200050228.
- Bart A. N., and Kyaw H. A. Survival of zebrafish, Brachydanio rerio (Hamilton-Buchanan), embryo after immersion in methanol and exposure to ultrasound with implications to cryopreservation. Aquac. Res., 34 (8), 609–615 (2003). DOI: 10.1046/j.1365-2109.2003.00852.x.
- Rahman S. M., Habib A., Khan A. R., Ahsan M., Arafat S. T., Rahman M., Alsaqufi A. S., Mathew R. T., Alrashada Y. N., and Alkhamis Y. A. Cryopreservation studies on silver carp (Hypophthalmichthys molitrix) embryos. CryoLetters, 42 (3), 178-187 (2021).
- Kopeika J., Zhang T., and Rawson D. Zebrafish embryos (Danio rerio) using microinjection. Cryo Letters, 27 (5), 319-28 (2006).
- Mazur P. Freezing of living cells: mechanisms and implications. Am. J. Physiol., 247, c125–c142 (1984). DOI: 10.1016/j.cryobiol.2005.04.008.
- Mazur P., Seki S., Pinn I. L., Kleinhans F. W., and Edashige K. Extra- and intracellular ice formation in mouse oocytes. Cryobiology, 51, 29–53 (2005). DOI: 10.1016/j.cryobiol.2005.04.008.
- Mazur P., Pinn I. L., and Kleinhans F. W. The temperature of intracellular ice formation in mouse oocytes vs. the unfrozen fraction at that temperature. Cryobiology, 54 (2), 223-33 (2007). DOI: 10.1016/j.cryobiol.2007.02.001.
- Ruffing N. A., Steponkus P. L., Pitt R. E., and Parks J. E. Osmometric behavior, hydraulic conductivity, and incidence of intracellular ice formation in bovine oocytes at different developmental stages. Cryobiology, 30, 562–580 (1993). DOI: 10.1006/cryo.1993.1059.
- Mazur P., and Kleinhans F. W. Relationship between intracellular ice formation in oocytes of the mouse and Xenopus and the physical state of the external medium—a revisit. Cryobiology, 56, 22–27 (2008). DOI: 10.1016/j.cryobiol.2007.10.002.
- Guenther J. F., Seki S., Kleinhans F. W., Edashige K., Roberts D. M., and Mazur P. Extra - and intra-cellular ice formation in stage I and II Xenopus laevis oocytes. Cryobiol., 52, 401-416 (2006). DOI: 10.1016/j.cryobiol.2005.04.008.
- Köseoglu M., Eroglu A., Toner M., and Sadler K. C. Starfish oocytes form intracellular ice at unusually high temperatures. Cryobiology, 43, 248–259 (2001). DOI: 10.1006/cryo.2001.2348.
- Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann. N. Y. Acad. Sci., 125, 658–676 (1965). DOI: 10.1111/j.1749-6632.1965.tb45420.x.
- Toner M., Cravalho E. G., and Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J. Appl. Phys., 67, 1582–1593 (1990). DOI: 10.1063/1.345670.
- Tikhomirov A.M., Ponomareva E.N., and Krasilnikova A.A. Use of plant and animal-derived lipids in deep freezing of fish oocytes. In Aquaculture: Development Prospects and Scientific Support of Innovative Technologies. International Scientific and Practical Conference, September 5–7, 2012 (Publishing House of RSAU-MTAA, Moscow, 2012a).
- Tikhomirov A.M., Ponomareva E.N., and Krasilnikova A.A. Development of a “coating-type” cryoprotectant model for cryopreservation of fish oocytes. In Aquaculture: Development Prospects and Scientific Support of Innovative Technologies. International Scientific and Practical Conference, September 5–7, 2012 (Publishing House of RSAU-MTAA, Moscow, 2012b).
- Tikhomirov A.M. Method for cryopreservation of sturgeon fish oocytes. Russian Patent No. 2460284, issued in 2012.
- Mayer I. The Role of Reproductive Sciences in the Preservation and Breeding of Commercial and Threatened Teleost Fishes. Adv. Exp. Med. Biol., 1200, 187-224 (2019). DOI: 10.1007/978-3-030-23633-5_7.
- Diwan A. D., Ayyappan S., Lal K. K., and Lakra W. S. Cryopreservation of fish gametes and embryos. Indian J. Anim. Sci., 80, 109–124 (2010).
- Bonisławska M., Formicki K., Korzelecka-Orkisz A., and Winnicki A. Fish egg size variability: biological significance. EJPAU, 4 (2), 02 (2001). Доступен по ссылке: http://www.ejpau.media.pl/volume4/issue2/fisheries/art-02.html
- Эрнст Л. К. и Сергеев Н. И. Трансплантация эмбрионов сельскохозяйственных животных (Агропромиздат, М., 1989).
- Otoi T., Yamamoto K., Koyama N., Tachikawa S., and Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology, 48, 769–774 (1997). DOI: 10.1016/s0093-691x(97)00300-2.
- Gorshkova A. G., Makarova N. P. i Dolgushina N. V. Dismorfizmy ootsitov v tsiklakh vspomogatel'nykh reproduktivnykh tekhnologiy. Akusherstvo i Ginekologiya, 6, 27–32 (2014). URL: medj.rucml.ru/journal/45562d4149472d41525449434c452d323437313735/ Article in Russian.
- Tervit H. R., Adams S. L., Roberts R. D., McGowan L. T., Pugh P. A., Smith J. F., and Janke A. R. Successful cryopreservation of Pacific oyster (Crassostrea gigas) oocytes. Cryobiology, 51 (2), 142-51 (2005). DOI: 10.1016/j.cryobiol.2005.06.001.
- Salinas-Flores L., Adams S. L., and Lim M. H. Cholesterol addition and removal in pacific oyster oocytes does not improve cryopreservation success. Cryo Letters, 29 (5), 391-398 (2008).
- Kalinina M. V., Tabel'skaya A. V. i Sukhin I. Yu. Rol' vneshnikh faktorov pri kul'tivirovanii tikhookeanskoi ustritsy Crassostrea gigas v pitomnike Primor'ya. 1. Vliyanie temperatury na skorost' razvitiya, rost i vyzhivaemost' embrionov i lichinok. Izvestiya TINRO, 203 (2), 427–442 (2023). Article in Russian.
- Khosla K., Zhan L., Bhati A., Carley-Clopton A., Hagedorn M., and Bischof J. Characterization of Laser Gold Nanowarming: A Platform for Millimeter-Scale Cryopreservation. Langmuir, 35 (23), 7364-7375 (2019). DOI: 10.1021/acs.langmuir.8b03011.
- Kroener C., and Luyet B. Formation of cracks during the vitrification of glycerol solutions and disappearance of the cracks during rewarming. Biodynamica, 10, 47–52 (1966).
- Fahy G. M., Saur J., and Williams R. J. Physical problems with the vitrification of large biological systems. Cryobiology, 27, 492–510 (1990). DOI: 10.1016/0011-2240(90)90038-6.
- Rabin Y., Steif P. S., Hess K. C., Jimenez-Rios J. L., and Palastro M. C. Fracture formation in vitrified thin films of cryoprotectants. Cryobiology, 53, 75–95 (2006). DOI: 10.1016/j.cryobiol.2006.03.013.
- Yavin S., and Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology, 67 (1), 81-9 (2007). DOI: 10.1016/j.theriogenology.2006.09.029.
- Solanki P. K., and Rabin Y. Temperature-dependent density and thermal expansion of cryoprotective cocktails. Cryo Letters, 43 (1), 1-9 (2022). DOI:10.54680/fr22110110112
- Rall W. F., and Meyer T. K. Zona fracture damage and its avoidance during the cryopreservation of mammalian embryos. Theriogenology, 31, 683–692 (1989). DOI: 10.1016/0093-691x(89)90251-3.
- Gangwar L., Phatak S. S., Etheridge M., and Bischof J. C. A guide to successful mL to L scale vitrification and rewarming. CryoLetters, 43 (6), 316-321 (2022).
- Williams R. J., and Carnahan D. L. Fracture faces and other interfaces as ice nucleation sites. Cryobiology, 27, 479–482 (1990). DOI: 10.1016/0011-2240(90)90036-4.
- Andreev A.A., Sadikova D.G., Ivlicheva N.A., and Boroda A.V. Formation of ice microparticles in cryoprotective solutions. Biophysics, 62 (2), 213–220 (2017).
- Boroda A. V., and Andreev A. A. Crack formation in cryoprotective medium at super low temperatures. Евразийское научное объединение (Eurasian Union of Scientists), 8 (30), 4-7 (2017).
- Feig J. S. G., Solanki P. K., Eisenberg D. P., and Rabin Y. Polarized light scanning cryomacroscopy, part II: Thermal modeling and analysis of experimental observations. Cryobiology, 73 (2), 272-81 (2016). DOI: 10.1016/j.cryobiol.2016.06.004.
- Luo D., Yu C., He L., Lu C., and Gao D. Development of a single mode electromagnetic resonant cavity for rewarming of cryopreserved biomaterials. Cryobiology, 53 (2), 288-93 (2006). DOI: 10.1016/j.cryobiol.2006.07.001.
- Fowler A. J., and Toner M. Prevention of hemolysis in rapidly frozen erythrocytes by using a laser pulse. Ann. N. Y. Acad. Sci., 858, 245–252 (1998). DOI: 10.1111/j.1749-6632.1998.tb10158.x.
- Jin B., Kleinhans F. W., and Mazur P. Survivals of mouse oocytes approach 100% after vitrification in 3-fold diluted media and ultra-rapid warming by an IR laser pulse. Cryobiology, 68 (3), 419-430 (2014). DOI: 10.1016/j.cryobiol.2014.03.005.
- Eisenberg D. P., Bischof J. C., and Rabin Y. Thermomechanical Stress in Cryopreservation Via Vitrification With Nanoparticle Heating as a Stress-Moderating Effect. J. Biomech. Eng., 138 (1), (2016). DOI: 10.1115/1.4032053.
- Alvarez C., Berrospe-Rodriguez C., Wu Ch., Pasek-Allen J., Khosla K., Bischof J., Mangolini L., and Aguilar G. Photothermal heating of titanium nitride nanomaterials for fast and uniform laser warming of cryopreserved biomaterials. Front. Bioeng. Biotechnol., 10, 1-14 (2022). DOI: 10.3389/fbioe.2022.957481.
- Song Y. C., Hagen P. O., Lightfoot F. G., Taylor M. J., and Smith A. C. In vivo evaluation of the effects of a new ice-free cryopreservation process on autologous vascular grafts. J. Invest. Surg., 13 (5), 279 – 288 (2000). DOI: 10.1080/08941930050206300.
- Brockbank K. G., Chen Z. Z., and Song Y. C. Vitrification of porcine articular cartilage. Cryobiology, 60 (2), 217–221 (2010). DOI: 10.1016/j.cryobiol.2009.12.003.
- Mikson K. B., Zinchenko A. V. i Bobrova E. N. Kriokonservirovanie embrionov v'yuna steklovaniem. V: Materialy mezhdunarodnoi konferentsii «Kriokonservirovanie geneticheskikh resursov, sovremennoe sostoyanie, problemy i perspektivy»; 2014 oktyabr' 28–30, Pushchino, Rossiya; 2014. с. 123–5.
- Maddock B. G. A technique to prolong the incubation period of brown trout ova. Prog. Fish Cult., 36, 219-222 (1974). DOI:10.20935/AcadBiol7280.
- Pullin R. S. V., and Bailey H. Progress in storing marine flatfish eggs at low temperatures. Rapp. P. Reun. Cons. Int. Explor. Mer., 178, 514-517 (1981).
- Garside E. T. Effects of oxygen in relation to temperature on the development of embryos of brook trout and rainbow trout. J. Fish. Res. Bd Can., 23, 1121-1134 (1966). DOI: 10.1139/f66-105.
- Elinson R. P. Fertilization in amphibians: the ancestry of the block to polyspermy. Int. Rev. Cytol., 101, 59–94 (1986). DOI: 10.1016/s0074-7696(08)60246-6.
- Edwards D. L., Mahony M. J., and Clulow J. Effect of sperm concentration, medium osmolality, and oocyte storage on artificial fertilisation success in a myobatrachid frog (Limnodynastes tasmaniensis). Reprod. Fertil. Dev., 16, 347–354 (2004). DOI: 10.10371/RD02079.
- Kouba A. J., and Vance C. K. Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod. Fertil. Dev., 21, 719–737 (2009). DOI: 10.1071/RD09038
- Uteshev V. K., Gakhova E. N., Kramarova L. I., Shishova N. V., Kaurova S. A., and Browne R. K. Refrigerated storage of European common frog Rana temporaria oocytes. Cryobiology, 83, 56–59 (2018). DOI: 10.1016/j.cryobiol.2018.06.004.
- Gagarinskiy E., Uteshev V., and Fesenko E. Jr. Prolonged hypothermic storage of oocytes of the European Common Frog Rana temporaria in a Gas Mixture of Oxygen and Carbon Monoxide. PLoS ONE, 18 (7), e0288370 (2023). DOI: 10.1371/journal.pone.0288370.
- Uteshev V. K., Gakhova E. N., Kramarova L. I., Shishova N. V., Kaurova S. A., and Penkov N. V. Refrigerated storage of oviductal oocytes into live females common frog Rana temporaria from 1 to 70 days. Russ. J. Herpetol., 31 (4), 239–245 (2024). DOI: 10.1016/j.cryobiol.2018.06.004
- Ruthsatz K., Dausmann K. H., Paesler K., Babos P., Sabatino N. M., Peck M. A., and Glos J. Shifts in sensitivity of amphibian metamorphosis to endocrine disruption: the common frog (Rana temporaria) as a case study. Conserv. Physiol., 8 (1), coaa100 (2020). DOI: 10.1093/conphys/coaa100.
- Smith G. D., Hopkins G. R., Mohammadi S., Skinner M. H., Hansen T., Brodie E. D. Jr., and French S. S. Effects of temperature on embryonic and early larval growth and development in the rough-skinned newt (Taricha granulosa). J. Therm. Biol., 51, 89-95 (2015). DOI: 10.1016/j.jtherbio.2015.03.010.
- Wersebe M., Blackwood P., Guo Y. T., Norman D. R., Earley R. L., and PFAFF A. S. The effects of different cold ‐ temperature regimes on development, growth, and susceptibility to an abiotic and biotic stressor. Ecol. Evol., 9, 3355–3366 (2019). DOI: 10.1002/ece3.4957.
License
Copyright (c) 2025 Наталья Шишова, Светлана Каурова, Виктор Утешев, Алексей Андреев, Эдит Гахова, Евгений Фесенко (Автор)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.