Viscosity of Ethylene Glycol Vitrification Solution in the Presence of Clathrate-forming Gases Xe and Kr Measured by Dynamic Light Scattering
- Affiliations
-
- 1. Institute of Cell Biophysics RAS FRC PSCBR RAS
- Published:
- 2024-11-07
- Keywords:
- cryopreservation, vitrification, vitrifying solution, gas hydrates, viscosity, xenon, dynamic light scattering
Abstract
The present state of the art in cryobiology is characterized by the intensive development of cryopreservation methods based on the vitrification of the aqueous phase. One of the actual problem is the search for pathways of decreasing the concentration of vitrification agents (cryoprotectants) to minimize the toxic effect on cells and tissues being frozen. In the present work, a new experimental approach has been developed that makes it possible to increase the viscosity of vitrification solutions by saturation with inert gases. An increase in viscosity is based on the hydrate formation, which occurs at low temperatures and leads to the transition of part of the water into the gas hydrate crystals. A decrease in the total content of water results in an increase in the concentration of the vitrification agent and, as a consequence, an increase in viscosity. The work experimentally confirms the increase in viscosity of a 56.4 vol.% aqueous solution of ethylene glycol, realized under conditions of increased pressure of Xe (9 atm.) and Kr (50 atm.) in the temperature range from -20 to -40°С. The viscosity under pressure was measured by the method of dynamic light scattering with some modifications.
Full text
Introduction
The present state of the art in cryobiology is characterized by the intensive development of cryopreservation methods based on the vitrification of the aqueous phase. The cooling at high rate to vitrification (glass transition) temperature makes it possible to minimize or completely eliminate the formation of ice crystals as the main damaging factor [1–3]. Because the cooling rate of macroobjects is limited by values of temperature conductivity, it is necessary to increase the viscosity of the medium for accomplishing the vitrification [4, 5]. The higher the viscosity of the medium, the slower the vitrified object can be cooled. Various cryoprotectants, such as ethylene glycol, propylene glycol, DMSO, and others are used as vitrification agents. These agents and their compositions used at high total concentrations (8-9 М) are highly toxic [6, 7]. Thus, for effective vitrification, the concentration of a cryoprotectant should be increased, whereas for the minimization of the toxic effect, it should be, on the contrary, reduced. It is worth noting that the toxicity of a cryoprotectants decreases with decreasing temperature; therefore, the saturation of an object with a cryoprotectant is performed in several stages by gradually decreasing the temperature from +20 to -20°С and simultaneously increasing the concentration. A decrease in the saturation temperature is limited by both its cold damage and a decrease in the rate the cryoprotectant diffusion, which affects its distribution throughout the object being cooled. Therefore, it is important to find a pathway for decreasing the concentration of a cryoprotectant at saturation temperature, simultaneously increasing it at lower temperatures in order to ensure the required viscosity.
We proposed that this can be achieved by binding a portion of water due to the formation of clathrate hydrates of inert gases, such as xenon, krypton, and argon [8]. For this purpose, a vitrification solution should be cooled under the pressure of hydrate-forming gases, and as the appropriate thermobaric conditions would be established, gas hydrates would form. During this process, the concentration of water in the solution of cryoprotectants would decrease, and viscosity would increase. Inert gases dissolved in the solution, in contrast to the majority of other substances affecting the viscosity, produce no toxic effect.
Each gas has specific thermobaric conditions for hydrate formation, and these conditions have been specified only for water and some aqueous solutions [9-11]. For vitrification solutions with a high content of cryoprotectants, they are unknown. It can only be stated that hydrate formation in these solutions will occur at temperatures substantially lower compared with water under the same gas pressures.
The goal of the work was (1) to develop a method for the real-time measurement of the viscosity of a solution under pressure at low temperatures; (2) to determine the thermobaric conditions for the formation of gas hydrates in solutions with a high content of vitrification agent; and (3) to verify the hypothesis that the viscosity of a vitrification solution increases under the pressure of an inert gas.
Materials and methods
Substances and conditions of measurements
The viscosity of an aqueous solution of ethylene glycol, one of the most commonly used cryoprotectors, under the pressure of krypton and xenon (NII КM, Russia, grade purity 4.0) was determined. Initially, an aqueous solution of ethylene glycol (Sigma-Aldrich, USA) of 56.4% (v/v) (a standard concentration for vitrification solutions) was used. Measurements were carried out at temperatures from +20 to -40°С. The pressure of krypton was 50 atm., and that of xenon 9 atm. The control experiment was carried out without applying pressure.
Method of measuring viscosity
To measure viscosity, the optical method of dynamic light scattering (DLS) was used, which was previously tested to measure the viscosity of carbon dioxide-saturated water [12].
The DLS method was initially developed for determining the size of particles in liquid media. It consists in measuring the autocorrelation function of the intensity of a scattered laser beam from the medium containing particles. Since particles are subject to Brownian motion, their local concentration and, consequently, the refraction index undergo fluctuations. The radiation scattered on these fluctuations of a refraction index is recorded at a particular angle, and the temporal autocorrelation function of intensity is determined. The temporal correlations depend on the particle diffusion coefficient. For a monodisperse size distribution of particles, the correlation function is related to the diffusion coefficient as follows:
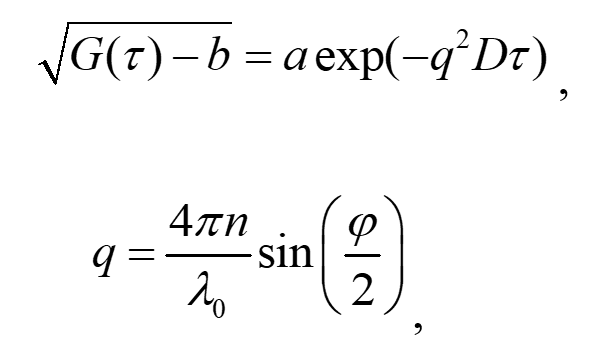 (1)
(1)
where τ is the delay time, a and b are the experimental constants, q is the scattering vector, D is diffusion coefficient of particles, l0 is the wavelength of laser radiation in vacuum, n is the coefficient of refraction of the liquid phase, and φ is the scattering angle.
Thus, the method makes it possible to determine the particle diffusion coefficient (more generally, the distribution of the diffusion coefficient) after which the hydrodynamic radius (R) of particles is calculated based on the Stokes-Einstein relation on assumption of their spherical shape:
R=kT/6πηD (2)
where k is the Boltzmann constant, T is the absolute temperature, η is dynamic viscosity. As it follows from formula (2), the size of a particle can be precisely determined provided that the exact value of dynamic viscosity is known. However, it is possible to formulate the inverse problem: use the measured values of the diffusion coefficients of particles of known size to calculate the viscosity of the medium using the formula:
η=kT/6πRD (3)
Since the DLS method is contactless, it is possible to measure the viscosity of the medium in a wide range of temperatures and pressures. The only condition is the optical transparence of the working chamber.
Construction of a thermobaric chamber for measuring viscosity
We designed a thermobaric chamber for measuring viscosity at various temperatures and pressures. A scheme of the chamber is shown in Fig. 1А. The body of the chamber is made from beryllium bronze. The optical window is made of leucosapphire in the form of a cylindrical ring with an internal diameter of 10 mm, an outer diameter of 30 mm, and a height of 10 mm. The space inside the ring is filled with the test solution being into which a laser beam is directed. Gas is delivered into the chamber under pressure through a steel tube. Pressure is measured with an accuracy of 0.5 atm by a manometer built into the tube immediately before the chamber. A thermocouple is inserted into the chamber at the top, which measures the temperature of the liquid with an accuracy of 0.1˚С.
Selection of reference particles for measuring the viscosity by the DLS method
As follows from formula (3), to measure the viscosity by the DLS method, it is necessary to use particles with a known size R. The particle material itself is not important in the context of the method used however, the following conditions should be met: (1) the distribution of particles should be close to monomodal; (2) particles should have aggregation stability throughout the experiment; (3) the particle size should be neither too great (less than 10 μm – the upper limit of the applicability of the DLS method), nor too small (more than 50 nm, to afford a high scattering intensity at small concentrations of particles); and (4) particles should not be chemically reactive toward medium in which they are dispersed. In this work, nanoparticles manufactured by the Biovaks (Russia) were used. The average particle radius claimed by the manufacturer is 52 ± 2 nm. The particle size determined by the DLS method was 53 ± 2 nm. The initial sample was a concentrated suspension of these particles in water. For generating enough scattering signal from particles, it was sufficient to add 1 vol% (percentage by volume) of the suspension into the solution being examined. The addition of 1 vol% of the suspension had no effect on the viscosity of the test solution.
Cooling system
The chamber was cooled using a specially designed system for the delivery of evaporating nitrogen into the jacket of the chamber holder. A scheme of the cooling system is shown in Fig. 1B. A tube with a heater was placed into a Dewar flask filled with liquid nitrogen. The space between the outer side of the tube and the neck of the Dewar flask was hermetically sealed. When the heater is switched on, nitrogen was intensively evaporated and was released under pressure through the tube connected to the chamber jacket. The stream of cold nitrogen was regulated by the voltage applied to the heater. An important feature of the system was that nitrogen leaving the cooling cuvette jacket was directed on the chamber window. This prevented water condensation on the cuvette window and increased the effectiveness of cuvette cooling, making it more uniform.
Viscosity measurement
Viscosity was measured using a Photocor Complex particle size analyzer (Photocor, Russia). A laser with a wavelength of 451 nm and a power of 25 mW was used. The cuvette was installed in a cuvette compartment of the device. Laser radiation scattered in a sample was recorded by an avalanche photodiode at an angle of 120˚ to the direction of the incident beam.
Viscosity was measured during the cooling of the solution being examined. The cooling rate was 0.5˚С/min. The time of one measurement was 2 min. Thus, the temperature change during a single measurement did not exceed 1˚С. The experiments were performed in 10 replicates.
For the control solution (56.4 vol% ethylene glycol at +20°С without external pressure), the viscosity was also measured using a standard vibro-viscometer SV-10 (A&D Company Limited, Japan). The experiment was carried out in 3 replicates.
Data analysis
The data analysis was conducted using SigmaPlot 12.5 software (Systat Software Inc, USA); the data were expressed as mean ± standard deviation. The significance of differences was determined using the Mann-Whitney U test. Values with p < 0.05 were considered statistically significant.
Results
Validation of viscosity measurements by the DLS method
To ensure the accuracy of the method used, the viscosity of a 56.4 vol% ethylene glycol solution at +20°С without external pressure was measured using the DLS method and a vibro-viscometer. The dynamic viscosity values obtained by both methods were the same, 4.8 ± 0.2 cP. In addition, the temperature dependence of the viscosity of a 56.4 vol% ethylene glycol solution without gas supply was compared with those reported in the literature [13] (Fig. 1С).
Viscosity of ethylene glycol solution under pressure of xenon and krypton
Figure 1D shows temperature dependences of the viscosity of a 56.4 vol% ethylene glycol solution under krypton and xenon pressure and without a gas supply. The minimal temperature to which it was possible to measure the viscosity was the temperature at which an active crystallization (presumably a mass hydrate formation) began. Under these conditions, large optical inhomogeneities appeared on the pathway of the laser beam, which did not allow further use of the DLS method. Since crystal formation (gas hydrate or ice) is a stochastic process, this occurred at different temperatures in a series of single-type experiments in the range from -25 to -40˚С.
Discussion
The accuracy of the viscosity measurement method utilizing DLS turned out to be comparable to that of the standard viscometric method. The alignment between the experimental values and the literature data shown in Figure 1B further supports the reliability of viscosity measurement using this approach. One significant advantage of the DLS method is its versatility, as it can be applied across a broad range of temperatures and pressures. Additionally, its effectiveness in measuring the viscosity of solutions containing biological macromolecules is noteworthy [14, 15].
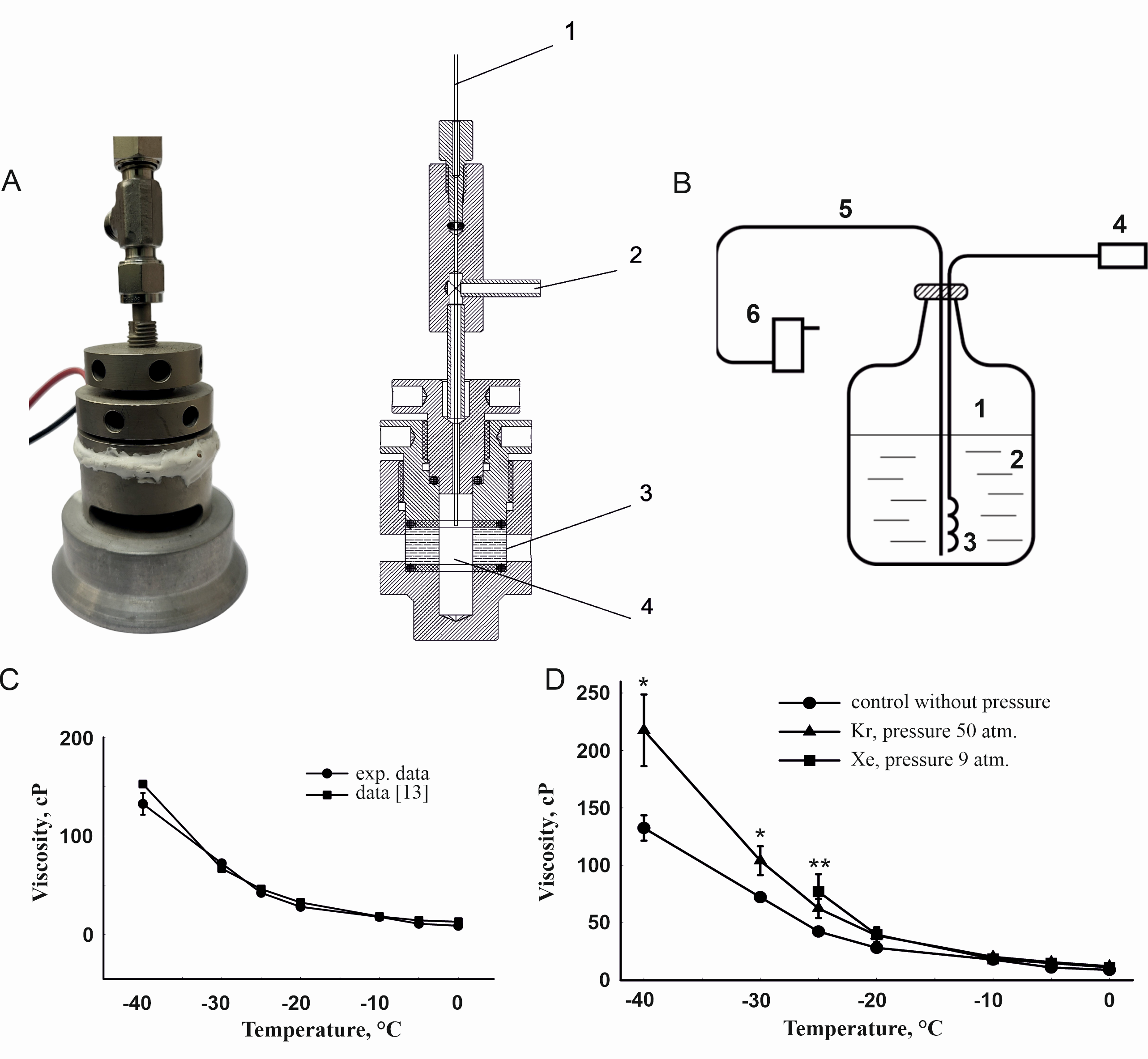
Figure 1. Dynamic viscosity of an aqueous 56.4 vol.% ethylene glycol solution under the pressure of xenon and krypton. A – Photo and scheme of a chamber for measuring the viscosity of medium by the DLS method under pressure with temperature control: 1 – thermocouple input, 2 – pressure gas pipe, 3 – leucosapphire optical window, 4 – test solution; B – System for cooling the thermobaric chamber: 1 – Dewar flask, 2 – liquid nitrogen, 3 – heater, 4 – heater control unit, 5 – heat insulation tube, 6 – chamber cooling jacket; C – temperature dependence of the viscosity of a 56.4 vol% ethylene glycol solution without gas supply, obtained by the DLS method (circle), and that reported in the literature [13] (square). D – The experimental values of dynamic viscosity of an aqueous 56.4 vol% ethylene glycol solution without gas pressure (circle), under a pressure of 9 atm. of xenon (square), and under a pressure of 50 atm. of krypton (triangle). Data are presented as mean ± standard deviation, n=10. Differences are significant (U-test) compared to the “control without gas pressure” group: *p<0.05 for the “Kr” group, **p<0.05 for the “Xe” group.
It follows from the dependencies presented in Fig. 1D that, as the temperature decreases to -20˚С and below, the viscosity of solutions being under pressure becomes higher than the viscosity of solutions without pressure. This can be explained by the formation of gas hydrates, which leads to a decrease in the water content in the solution and, correspondingly, an increase in the concentration of ethylene glycol in solution; as a result, the viscosity of the solution increases. The viscosity under the pressure of gases increased by 60% compared with the viscosity in the control at -40°С.
To use this phenomenon in cryopreservation, it is important to determine to what degree the concentration of a cryoprotectant in solution under the pressure of a gas can be decreased to obtain the same viscosity as that of the control solution without gas. The gas supply increases the viscosity of the 56.4 vol% ethylene glycol solution as much as if the concentration of ethylene glycol was increased to 60 vol% – by 3.6 vol%. As is known, the dependence of the solution viscosity on the cryoprotectant concentration is not linear, its growth accelerates with increasing concentration [13]. Therefore, a decrease in the concentration of cryoprotectant below 56.4 vol%, which can be compensated by the supply of a hydrate-forming gas, is slightly more than 3.6%. According to our estimates, we are talking about a permissible decrease in the concentration of ethylene glycol by about 4,5%. In terms of toxicity, this is a notable decrease in the cryoprotectant concentration. It is important that the increase in cryoprotectant concentration as a result of hydrate formation occurs at low temperatures. Therefore, this effect should not lead to an increase in toxicity. Conversely, as the temperature rises, the hydrate decomposes and the water content in the solution increases, which should reduce toxicity due to dilution of the toxic agent.
In the study of the effect of inert gases on the viscosity of vitrifying solutions, we limited ourselves to examining xenon and krypton. The pressures were chosen to ensure conditions for hydrate formation at experimentally attainable temperatures (above -40˚С). However, at lower pressures, it is possible to decrease the temperature of hydrate formation. Along with xenon and krypton, argon can also be of interest. Its distinguishing feature is that, for the formation of hydrates, either substantially higher pressures or substantially lower temperatures are required. The idea of utilizing a mixture of hydrate-forming gases is also quite intriguing.
Conducting experimental research has enabled us to identify several critical issues that need to be addressed within the framework of the proposed approach to optimizing vitrification methods. For instance:
- May it be that the resulting gas hydrate crystals will become the nucleator for ice crystals, which will make the vitrification of solutions impossible? There are reasons to suggest that crystal structures of gas hydrates and ice are fundamentally different, therefore, gas hydrate cannot serve as ice nucleator, and vice versa. Artyukhov and coworkers simulated the behavior of an aqueous xenon solution at different temperatures and pressures [16]. The simulation showed that, in the presence of ice in the aqueous solution, the nucleation of crystal hydrates occurs not at the water/ice interface but within the thickness of the liquid phase. Similarly, the nucleation of ice crystals occurs independently of gas hydrate. Experimental confirmation of these simulations are the results of Nesterov who recorded a persistent coexistence of the crystals of propane hydrate and supercooled water during the dissociation of propan gas hydrates [17]. If propan hydrate crystals were effective ice nucleators, their persistent coexistence would be impossible.
- What destructive effect will the resulting gas hydrate crystals have? Presumably, as in the case of ice crystals, the main factor influencing the intactness of the object being frozen will be the size of these crystals. According to some estimates, this size must not exceed 300–400 nm [18]. Theoretically, during the formation of gas hydrates, there are more opportunities to affect the formation of crystals, in particular, their size than when ice crystals form. To the conventional choice of the temperature regime can be complemented by pressure regime, the ratio of water and gas amounts, the use of gas mixtures, and other factors. When choosing the vitrification conditions, it is necessary to avoid crystallization (presumably mass hydrate formation) similar to what was recorded at terminal stages of cooling in our experiments.
- In this paper, we considered the issue of increasing the viscosity of a cryoprotectant solution due to the formation of gas hydrates. The cooling rates were chosen low enough (0.5°C/min) to enable reliable measurement of viscosity and demonstrate effects that can be used for vitrification. When directly implementing the vitrification procedure, apparently, the cooling rates should be higher, which will allow the crystal formation process to shift to a lower temperature region and further reduce the cryoprotectant concentration. Therefore, the issue of choosing optimal cooling rates, initial concentrations of cryoprotectants and cryoprotectants themselves remains open.
The experimentally justified answer to these questions will be the subject of further research.
Conclusion
An increase in the viscosity of a 56.4 vol% ethylene glycol solution under the action of increased pressure of Xe and Kr has been experimentally demonstrated. Approaches to using this effect in the development of cryopreservation methods based on vitrification have been substantiated. A method for “real time” recording the viscosity of cryoprotective solutions based on dynamic light scattering has been developed.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation in the framework of the State assignment of PSCBR RAS №075-00609-24-01.
Acknowledgments
The preparation of this publication was overshadowed by the death of our co-author, the talented engineer Nikolai Eduardovich Shvirst. We dedicate this work to his memory.
We express our sincere gratitude to L.I. Kramarova, an employee of the ITEB RAS, for her assistance in preparing the article.
The work was conducted using equipment from the Optical Microscopy and Spectrophotometry Sector of Center of the Shared Use PSCBR RAS.
References
- Stiles W. On the cause of cold death of plants, Protoplasma 9, 459–468, (1930). DOI:10.1007/BF01943364
- Luyet B. The vitrification of organic colloids and of protoplasm, Biodynamica 1, 1–14, (1937)
- Fahy G.M., Wowk B. Principles of Cryopreservation by Vitrification, in: Methods Molecular Biology: Cryopreservation and Freeze-Drying Protocols, Ed. by W.F. Wolkers & H. Oldenhof (UK, Humana Press, 2015) pp.305-320
- Gonzales F., Luyet B. Resumption of heart beat in chick embryo frozen in liquid nitrogen, Biodynamica 7, 1–5, (1950)
- Fahy G.M., Hirsh A. Prospects for organ preservation by vitrification, in: Organ preservation, basic and applied aspects, Ed. by D.E. Pegg, I.A. Jacobsen, N.A. Halasz (Lancaster, MTP Press, 1982) pp. 399–404.
- Davidson A.F., Glasscock C., McClanahan D.R., Benson J.D., Higgins A.Z. Toxicity Minimized Cryoprotectant Addition and Removal Procedures for Adherent Endothelial Cells, Plos One 10, e0142828, (2015). DOI: 10.1371/journal.pone.0142828
- Best B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions, Rejuvenation Res 18, 422-436, (2015). DOI: 10.1089/rej.2014.1656
- Shishova N. V., Fesenko E. E. (Jr). The Prospects of the Application of Gases and Gas Hydrates in Cryopreservation, Biophysics 60, 782–804, (2015). DOI:10.1134/S0006350915050218
- Istomin V.A., Yakushev V.S. Gas Hydrates at Natural Conditions, (Nedra, Moscow, 1992 (in Russian).
- Dyadin Yu.A., Larionov E.G., Mirinskij D.S., Mikina T.V., Aladko E. Ya, Starostina L.I. Phase Diagram of the Xe–H2O System up to 15 kbar, Journal of Inclusion Phenomena and Molecular Recognition in Chemistry 28, 271–285, (1997). DOI:10.1023/A:1007911123739
- Ohgaki K., Sugahara T., Suzuki M., Jindai H. Phase behavior of xenon hydrate system, Fluid Phase Equilibria 175, 1–6, (2000). DOI:10.1016/S0378-3812(00)00374-5
- Uchida T., Ohmura R., Nagao J., Takeya S., Ebinuma T., Narita H. Viscosity of Aqueous CO2 Solutions Measured by Dynamic Light Scattering, Chem. Eng. Data 48, 1225-1229, (2003). DOI: 10.1021/je034041x
- Ethylene Glycol Product Guide. Доступен по ссылке: https://www.meglobal.biz/wp-content/uploads/2024/03/MEG_Guide_Rev_2024_Wpdf (дата посещения: 07.07.2024).
- He F., Becker G.W., Litowski J.R., Narhi L.O., Brems D.N., Razinkov V.I. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal Biochem. Apr 1;399(1),141-3, (2010). DOI: 10.1016/j.ab.2009.12.003
- Gilroy EL, Hicks MR, Smith DJ, Rodger A. Viscosity of aqueous DNA solutions determined using dynamic light scattering. Oct 21;136(20),4159-63, (2011). DOI: 10.1039/c1an15475c
- Artyukhov V. I., Pulver A. Y., Peregudov A., Artyuhov I. Can xenon in water inhibit ice growth? Molecular dynamics of phase transitions in water-Xe system, Chem. Phys. 141, 034503, (2014). DOI: 10.1063/1.4887069
- Nesterov A.N. Kinetics and mechanism of hydrate formation of gases in the presence of surfactants. Diss. for the degree of Doctor of Chemical Sciences (Institute of the Earth's Cryosphere SB RAS, Tyumen, 2006).
- Takahashi T., Hirsh A., Erbe E., Williams R.J. Mechanism of cryoprotection by extracellular polymeric solutes, J. 54, 509–518, (1988). DOI: 10.1016/S0006-3495(88)82983-7.
License
Copyright (c) 2024 Пеньков Н. В. , Крассова Н.Е. , Швирст Н.Э., Фесенко Е.Е. (мл.) (Автор)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.