Modern Biotechnologies in Crop Production: Achievements and Development Trends
- Affiliations
-
- 1. The All-Russian Collection of Microorganisms, G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms at the Federal Research Center "Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences"
- Published:
- 2024-11-07
- Keywords:
- agrobiotechnology, selection, genetically modified plants, biofertilizers, biopesticides
Abstract
The review is devoted to the basic cutting-edge crop production technologies aimed at obtaining high-quality and safe products, as well as the technologies able to reduce yield and productivity losses caused by pathogens and pests. The advantages and disadvantages of classical approaches to breeding plants, namely creation of new and improvement of the existing varieties through selection and hybridization, and genetic modification of plants are discussed. The genetic certification of agricultural crops, the use of biofertilizers and biopesticides, as well as the development of effective test systems for diagnosing plant diseases are considered.
Full text
Introduction
Most experts consider the growth of the world population inevitable: as of May 2024, the Earth's population makes up 8.108 billion people [1]; according to various forecasts, it will grow to 8.5 billion by 2030 and reach almost 10 billion by 2050 [2]. The population growth and the increasing average level of well-being raise the food demand, which is however highly heterogeneous. The UN reports show that while 30% of the world's population lacks access to adequate food and about 9 million people die from hunger every year, 30% of all food in the world is wasted [3-4]. At the same time, according to existing estimates, if the current intensity of carbon emissions is maintained over the next 25 years, there will be a global decline in agricultural productivity [5], which threatens significant changes in agroclimatic conditions and the sanitary and epidemiological situation, including the spread of epiphytotics to new territories.. Scientific and technological progress and the sixth technological order, the basis of which is nanotechnology, are called upon to influence such challenges to the development and even existence of mankind. At the intersection of biology and nanotechnology, a new field of science has emerged – nanobiotechnology aimed at the research of nanoobjects of a biogenic nature: DNA, RNA, antigens, antibodies, enzymes and other biological macromolecules. Developments in the field of nanobiotechnology find practical application in medicine, food industry, energy, environmental protection and agriculture. Currently, the main challenge for agricultural biotechnology is to reduce yield and productivity losses caused by pathogens and pests, and to obtain safe products [6].
There are four main ways to solve this problem:
- The use of chemicals (fertilizers, pesticides, drugs, additives) leads to environmental pollution, and due to biomagnification, the concentration of toxic substances can increase at each trophic level in the food network.
- Application of biological plant and animal protection means.
- Classical approach aimed at improving existing plant varieties and creating new ones mainly through selection and hybridization. However, this is a long process, that may take decades.
- Genetic modification, which is a targeted change in the genotype including transgenesis, cisgenesis, subgenesis, and intragenesis.
In addition, for this purpose, technologies for microclonal propagation of plants, substrate-free technologies for their cultivation (hydro- and aeroponics), city farming, the use of unmanned aerial vehicles in agriculture for sowing seeds, applying fertilizers and plant protection products, as well as remote monitoring of the condition of plants and soil have been developed and are being actively implemented.
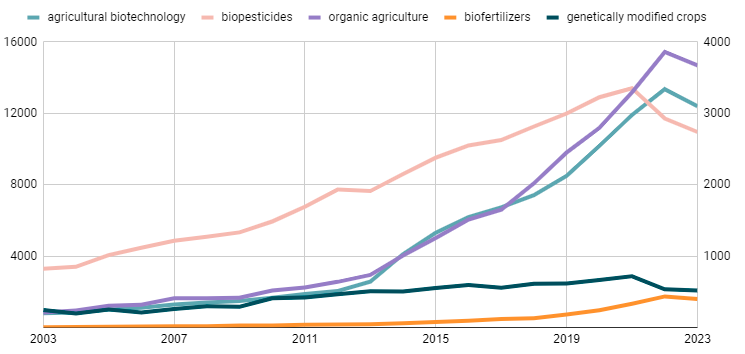
Figure 1. The number of published articles from 2003 to 2023, based on the keywords "agricultural biotechnology" (left vertical axis) and "biopesticides", "biofertilizers", "genetically modified crops" and "organic agriculture" (right vertical axis). The data was obtained from the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) on May 8th, 2024.
There is no doubt that both agricultural biotechnology in general and its individual areas have attracted significant interest from the scientific community in recent years (Figure 1), which is being formed under the influence of global challenges of our time and humanity’s desire for sustainable development.
Russia occupies a leading position in the world in terms of agricultural land area and is among the top five countries in terms of arable land area and reserves of natural resources, thus possessing unique natural conditions to ensure its own food security. Theory and practice of agricultural biotechnology are, therefore, focused on the sustainable development of agro-industrial complexes (AIC), production of high-quality and safe food, processing of agricultural waste, and restoration of soil fertility. In plant growing, this is the breeding of new plant varieties with improved nutritional properties and resistance to adverse environmental factors (drought, salinity, pollution, etc.); genetic certification; development of biological means to combat weeds, phytopathogens and pests; production of biofertilizers; recycling of waste and by-products.
Genetic breeding technologies to increase the yield, quality, sustainability
and safety of agricultural crops
Selection and genome editing
Since ancient times, people have sought to improve the qualitative and quantitative properties of agricultural crops. To do this, they consistently selected the best fruits, the largest seeds and grains, gradually changing plants in the right direction thus carrying out artificial selection. The basis of such phenotypic selection was the wide variability of the source material and the repeated sorting of forms in accordance with the visual and organoleptic preferences of man. With the discovery of the foundation of genetics - the principles of transmission of hereditary characteristics from parent organisms to their descendants, known as Mendel's laws, it became possible to consciously and scientifically control the transmission of target characteristics. It became clear that a simple selection alone was not capable of imparting new qualities and properties to cultivated plants. To obtain new varieties of plants, crossing organisms with the necessary characteristics (hybridization) became widespread. Despite the fact that plant breeding has been successfully used from the very beginning of human civilization to this day, the process of creating new varieties remains very labor-intensive and requires many years of hard work. The main methods of selection in crop production are mass and individual selection, intraspecific and distant hybridization, inbreeding (closely related crossing of plants), polyploidy and experimental mutagenesis using radiation or chemicals [7]. For cross-pollinated plants, mass selection of individuals with the necessary properties is most common. As a result, new varieties are obtained that are not genetically homogeneous. If it is necessary to obtain a genetically homogeneous variety (pure line), then individual selection is used, in which offspring are obtained from one single individual with the necessary characteristics using self-pollination. Self-pollinating populations maintain a certain level of genetic variation. Experimental polyploidy is also widely used in plant breeding, i.e. a multiple increase in the number of chromosomes in a eukaryotic cell. Polyploids are characterized by fast growth, large size and high yield; successful examples of this method are triploid sugar beet and seedless watermelon, tetraploid clover, buckwheat, rye, corn, and durum wheat, as well as hexaploid bread wheat. Polyploids are obtained by artificial mutagenesis using chemicals, such as colchicine, which destroy the spindle and the duplicated chromosomes do not diverge and remain in one nucleus. On the basis of phenotypic selection, two new areas of plant biotechnology appeared, namely cellular and gametic-zygotic selection [8]. In cell selection, lines and plants with valuable hereditary traits are selected at the level of cells cultured in vitro. The undeniable advantage of this approach is the ability to work with millions of plant cells during targeted selection in Petri dishes with the subsequent plant regeneration. This facilitates and speeds up the traditional breeding process aimed at creating new plant varieties.The main tools are technologies for microclonal propagation of distant hybrids, in vitro fertilization, cultivation of ovules and immature hybrid embryos, somatic hybridization, etc. The main goal of gametic-zygotic selection is the search for desired recombinants at the post-meiotic stages of the organism development by cultivating anthers and microspores. However, modern agriculture is experiencing an urgent need to quickly obtain new varieties of agricultural crops that are resistant to the effects of stressful biotic and abiotic environmental factors, have high productivity, long shelf life of crops and other characteristics.The wild ancestors of cultivated plants possess many of the necessary qualities. In order to transfer their target genes to modern varieties, it is necessary to carry out interspecific crossing, which is technologically more difficult and is not even always realizable due to genetic incompatibility. The development of genetic engineering, which made it possible to transfer genes from one organism to another and gave rise to the development of genomic selection, became a solution to this problem. The main methods of biotechnological modification of the plant genome, in addition to physical or chemical artificial mutagenesis, are transgenesis, cisgenesis, intragenesis, and subgenesis [8]. Transgenesis is the introduction of a foreign gene, called a transgene, from an unrelated organism. Cisgenesis is the introduction of a gene from a closely related species with which a sexual intercourse is potentially possible in nature. The result of this method is absolutely similar to classical breeding work, but the whole process takes much less time and also prevents the penetration of unwanted linked genes from the donor plant to the recipient’s genome [9]. Intragenesis is the introduction of an own gene, for example, to improve the properties of a plant by introducing an additional copy of its own gene into its genome in order to achieve its over-expression, or “shutdown”. In subgenesis, genetically modified (GMO) plants can be created using gene knockdown, which reduces the expression of one or more genes by changing the corresponding nucleotide sequence or using a short oligonucleotide complementary to the corresponding mRNA molecule, or gene knockout (gene inactivation/deletion).
To obtain genetically modified organisms (GMO), bioengineering technologies such as gene guns, electroporation, microinjections, agrobacteria and more precise genome editing tools are used. The gene gun implements bioballistics technology and is designed as an air pistol that fires tungsten, silver or gold particles coated with reporter genes. The particles penetrate the cell wall and organelle membranes, the DNA is then separated from the metal and is incorporated into the DNA of target cells, such as callus. This method was successfully applied to many plant crops, especially monocots such as wheat or corn, for which transformation using Agrobacterium tumefaciens (= Rhizobium radiobacter) was less successful. In addition, the method is used to deliver DNA vaccines. The main disadvantages of this method include the high proportion of damaged target cells.
The alternative methods include microinjection, where foreign DNA is directly injected into target cells, and electroporation, where foreign DNA enters plant cells through miniature pores that are temporarily created by electrical pulses [10]. However, the previously most common method of transforming crop plants (transfection) was mediated by the phytopathogenic bacterium A. tumefaciens capable of ahorizontal gene transfer resulting in the proliferation of modified plant cells at the soil level (crown gall). The genetic information necessary for tumor growth is encoded in a mobile circular DNA fragment, Tiplasmid [11]. When an agrobacterium infects a plant, it transfers a section of the Ti plasmid, known as T-DNA, to a random location in the plant's genome. It is this ability that is used in genetic engineering to improve the properties of plants. T-DNA is removed from the bacterial plasmid to leave only two small (25 base pairs) edge repeats. The genes, to be introduced into the plant cell, are cloned into a special vector for plant transformation, which consists of a T-DNA section of a neutralized plasmid and a selective marker, for example, an antibiotic resistance gene. The latter allows for selecting plants in which transfection has occurred: those that have incorporated T-DNA and the resistance gene in their genome are able to grow in a medium supplied with an antibiotic, the others die. This method is particularly successful when applied to dicots such as potatoes, tomatoes, and tobacco, but is less efficient with monocots. The main disadvantage of agrobacterial transformation is the inability to determine the place in the plant DNA where a new construct will be inserted. This problem is successfully solved by the latest methods of genome editing based on the use of peculiar enzymatic systems - programmable nuclease platforms or “molecular scissors”. These enzymes create site-specific double-strand DNA breaks in a specific region of the genome, which are then repaired through recombination, allowing targeted mutations. Currently, 4 types of endonucleases are used: meganucleases, zinc finger nucleases, TALEN nucleases, and the CRISPR-Cas system [12].
Meganucleases are the most specific naturally occurring restriction enzymes, characterized by a large recognition site of 12 to 40 bp that typically occurs only once in the genome. The advantage of meganucleases, in addition to high accuracy, is the formation of highly recombinogenic 3′-OH-overhanging ends during cutting. In addition, genetic recombinations induced by meganucleases are limited by their finite amount available. Despite the natural existence of hundreds of meganucleases in pro- and eukaryotic cells and the fact that each of them is able to tolerate minor variations in its recognition site, the likelihood of finding a meganuclease capable of cutting a given gene at a specific site is quite low. By correcting the low natural diversity of these enzymes, biologists artificially construct engineered analogues with hybrid or altered specificity.
ZFN (Zink Finger Nucleases) or nucleases based on proteins with a zinc finger domain are artificial restriction enzymes that are obtained by cross-linking two dissimilar domains: the DNA-binding domain of the zinc finger, which recognizes from 9 to 18 bp, and the nuclease one that is responsible for DNA cleavage. The first consists of tandem microdomains, the structure of which is stabilized by zinc ions. These “fingers” are widespread in eukaryotes and ensure the specific interaction of the proteins they carry with DNA and other molecules, most often regulating transcription. Each of the “fingers” is linked to the nonspecific cutting nuclease domain of the FokI restriction enzyme. Such proteins are easier to construct than meganucleases, but it is difficult to select “fingers” for all the desired combinations of nucleotides and ensure the correct relative position of the two ZFNs. In addition, if zinc finger domains are not specific enough for their target site or they do not target a unique site in the genome of interest, the off-target cleavage may occur. In this case, a sufficient number of double-strand breaks are formed, which inhibits the repair mechanism and, as a consequence, leads to chromosomal rearrangements or cell death.
TALEN (Transcription Activator-Like Effector Nuclease) are artificial nucleases based on effectors similar to transcription activators. They work in pairs and consist of a FokI nuclease domain and a TALE DNA-binding domain. Natural DNA-binding domains TALE are also involved in the regulation of gene expression, but only relate to virulence factors of plant pathogens from the genus Xanthomonas. They bind to promoters in plant cells and change the work of genes in ways that suppress resistance to infection. TALE are composed of tandem repeats of amino acid modules, each recognizing 1 bp. These endonucleases are easy to construct, fairly accurate, and less cytotoxic than ZFN.
The CRISPR-Cas system is an adaptive immunity system of bacteria and archaea, which is based on short palindromic repeats (CRISPR, clustered regularly interspaced short palindromic repeats), regularly arranged in groups and separated by unique sequences (spacers). Spacers are borrowed from foreign genetic elements that the cell encountered in the past. RNA, transcribed from CRISPR loci, together with associated Cas proteins, provide adaptive immunity due to the complementary binding of RNA to the nucleic acids of foreign elements and their subsequent detection by Cas proteins. If a fragment of the virus is “recorded” in the CRISPR RNA spacer, Cas proteins cut the foreign DNA and destroy it. CRISPR-Cas techniques have been used for targeted editing of genomes by various research groups since 2013. And in 2020, for achievements in this promising area of genetic engineering, the Nobel Prize in Chemistry was awarded to scientists E. Charpentier and D. Doudna for developing a method of genome editing. More details about CRISPR/Cas systems for plant genome editing can be found in the review by Mikhailova et al. [13].
Figure 2 summarizes the results of genetic breeding technologies for the domestication of some of the most important agricultural crops (tomato, potato, rice, corn, and wheat) through artificial selection and their further genomic editing, with examples of specific GM varieties [14].
Genetically modified crops: examples, benefits and risks
Genome editing is the most modern tool for selecting organisms with desired properties. One of the main advantages of GMO products for the agricultural sector is their economic value. For example, according to PG Economics, GMO crops increased the income of farmers worldwide by $14 billion in 2010, with more than half of this amount coming from agriculture in developing countries [15]. Taking into account the fact that 80% of the world's agricultural land is allocated for the production of animal feed, and most of the annually grown GMO crops are used to feed livestock and poultry, the growing demand for meat leads to an increase in the demand for GMO feed crops [16]. A meta-analysis of the agronomic and economic indexes of three major GMO crops (soybean, corn, and cotton) over a nearly 20-year period revealed quite low production costs in case of herbicide-tolerant crops, while with pest-tolerant crops, the reduction of the amount of pesticides was offset by higher prices for their seeds, thus making the overall production costs approximately the same [17]. With GMO crops, farmers saw a 69% increase in profits, with a 9% increase in yield for herbicide-resistant varieties and a 25% increase for insect-resistant varieties [17]. Another study showed that knockout of the KRN2 genes in corn and OsKRN2 in rice using CRISPR genome editing technology increased grain yield by ~10% and ~8%, respectively, without any identified negative effects [18].
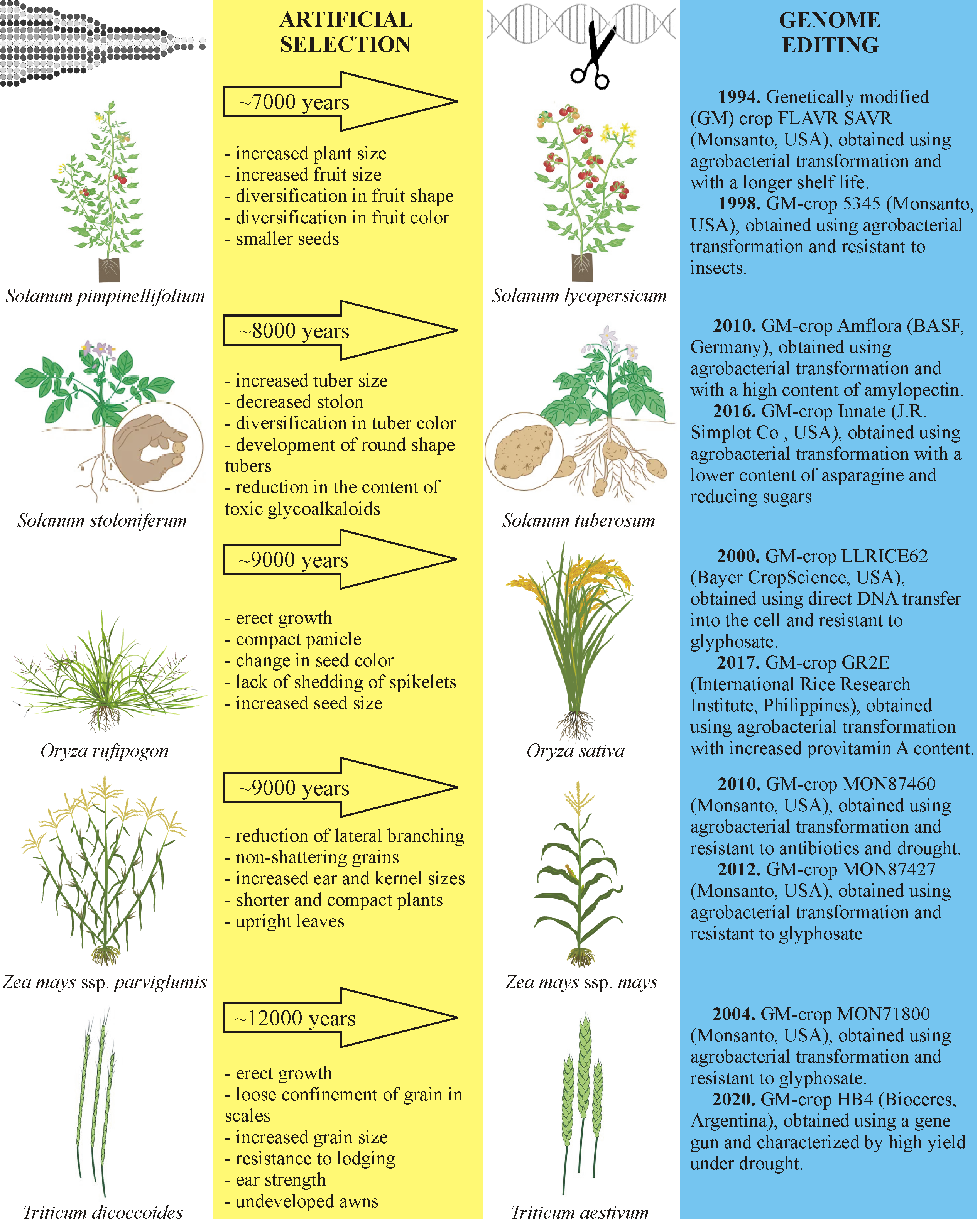
Figure 2. The characteristics of tomato, potato, rice, corn, and wheat, obtained through artificial selection and genome editing.
The combination of such advantages of the use of GMO in agriculture as the increased productivity, reduced use of land resources, decreased amounts of fertilizers and pesticides, and reduced use of agricultural machinery has not only an economic but also an environmental effect, since it decreases carbon emissions in the agricultural sector: according to some estimates, by 7.5% of total EU agricultural emissions or 33 million tons of CO2 [19].
There is now scientific consensus that currently available GMO food products do not pose a greater risk to human health than conventional foods obtained using traditional methods [20–21]. However, any GMO product must be tested on a case-by-case basis before introduction [22]. Genetic experiments are being conducted all over the world, resulting in improved varieties of agricultural plants, namely:
- GMO plants with improved nutritional properties – biofortification. They are, for example, wheat, rice, legumes, sweet potatoes, corn and, microgreens fortified with zinc; rice, legumes, and vegetables fortified with iron; sorghum and cassava, enriched with amino acids and proteins; soybean enriched with polyunsaturated fatty acids [23]; sweet potatoes, corn and cassava fortified with carotenoids; GMO - “golden rice” with increased beta-carotene content [24]; tomatoes with increased lycopene [25]. These crops can be used both directly as food and as animal feed. For example, the nutritional value of the oilseed and fodder crop of Camelina sativa has been enhanced by genetic modification to accumulate high levels of long-chain omega-3 polyunsaturated fatty acids [26]. In addition, the development is underway to improve the taste qualities of GMO plants, for example, the creation of seedless tomatoes [27]. Another aspect of this area is the safety of agricultural crops, i.e. genetic modification of plants, which can decrease the content of natural toxins in products. For example, GMO cassava, a tropical edible tuber plant, contains less cyanogenic glycosides and an increased content of protein and other nutrients [28]. Another example is GMO potatoes with a reduced content of the amino acid asparagine. This amino acid turns into carcinogenic acrylamide when potatoes are fried.
- Plants with a longer shelf life, better marketable condition or different growing requirements. For example, methods for genome editing of tomato were developed to increase their shelf life [29-30]. In commercial varieties of Arctic apples, the expression of the gene responsible for the synthesis of polyphenol oxidase is suppressed; this enzyme causes the fruits darken when cut [31]. Genetic modifications of potatoes were proposed to make them more attractive in the production of chips [32]. There are also genetic modifications of some crops, e.g. tomatoes [33], to create a more compact form for growing them in urban environments (vertical farms).
- Crops that are resistant to drought, salinity, pollution and other adverse stress factors. Examples of successful development of such crops include the creation of a salinity-resistant peanut crop, genetically modified by introducing the AtNHX1 ion transporter gene through agrobacterial transformation; this gene binds excess sodium ions into a large intracellular vacuole [34]. Another example is related to bioengineering studies of plant resistance to drought, which is achieved by modifying genes responsible for crassulic acid metabolism or CAM photosynthesis [35]. This mechanism was developed by many xerophytic plants in the course of their adaptation to arid conditions and by tropical epiphytes to a lack of water. This work is especially relevant for such water-demanding crops as rice, wheat, and soybeans. Several mechanisms of salinity tolerance have been identified in salt-tolerant crops.
- Crops with multiple resistance to phytopathogens and pests. One popular way to create insect-resistant GMO plants is to use the entomopathogenic bacterium Bacillus thuringiensis (Bt) as a source of endotoxin genes [36]. The introduction of Bt crops in the United States between 1996 and 2005 reduced overall insecticide use by almost 20% [37]. Virus-resistant GMO varieties of papaya, potatoes, corn, squash, and pumpkin have been created.
- GMO plants resistant to pesticides. The development of crops tolerant to herbicides such as glyphosate [38–39 and dicamba [40] has significantly improved agricultural efficiency worldwide.
- High-yield GMO crops with controlled photosynthesis. Modification of some genes involved in photoprotective mechanisms increased tobacco yield by 15% [41]. At the same time, the plants differed from the control ones in higher growth, large leaves and a massive root system. Another improvement in photosynthesis concerns photorespiration in C3 plants, which fix carbon dioxide directly from the air, rather than after release from malate as in C4 and CAM plants. The incorporation of C4 pathway into C3 plants can double the yield of grain crops such as rice [42–43].
- Crops grown for the production of pharmaceuticals and feed for the needs of industrial biotechnology (vaccines, antibodies, bioadditives for improving the quality of feed, amino acids, feed protein, enzymes, vitamins, probiotics, veterinary biological products), for food biotechnology (starch and glucose-fruit syrups; enzymes and microorganisms for dairy, oil-fat and meat processing industries; organic acids such as citric, lactic, acetic and others; products of advanced processing of food raw materials, bioplastics), for the production of biofuel from waste in the bioenergy industry.
- GMO plants that improve the environmental situation in the world. For example, the use in agriculture of genetically modified plants with improved carbon biosequestration capabilities. An example of the latter case is the Harnessing Plants Initiative (HPI) of the Salk Institute for Biological Research (USA) to create GMO plants with increased root mass and depth of root penetration as well as with a high content of the hard-to-degrade biopolymer suberin. This polymer is part of the cell walls which will bind atmospheric carbon (https://www.salk.edu/harnessing-plants-initiative/).
Genetic certification of agricultural crops
Genetic certification of an agricultural crop implies the creation of an official document about its taxonomic affiliation, close relationships, and characteristic features that are steadily transmitted from generation to generation [44]. To preserve the diversity of crop varieties, it is recommended to develop approaches to their documentation in accordance with the International Code of Nomenclature for Cultivated Plants [45]. In accordance with the Code, the nomenclatural standard of a variety, represented by a herbarium specimen, establishes its name and helps to avoid duplication. According to the established procedure, the author of the variety or a representative of the institution where the variety was bred transfers plant material with appropriate documentation to the scientific herbarium for registration and preservation of nomenclature standards [45]. For genotyping of plant varieties, various DNA markers are used, i.e. polymorphic traits detected by molecular biology methods at the level of the DNA nucleotide sequence for a specific gene or for any other part of the chromosome when comparing the genotypes of different individuals, populations, varieties, subspecies, and species.
The following technologies are used to identify and evaluate genetic resources of cultivated plants [46]:
- Probe-based DNA markers. For example, a method for studying Restriction Fragment Length Polymorphism (RFLP) using blot-hybridization. This method involves isolating DNA from the target object, cutting the DNA using restriction endonucleases, electrophoretic separation of the resulting fragments (restricts), and hybridization of specific DNA probes with the resulting restricts. Combinations of restriction enzymes and DNA probes provide highly specific polymorphic spectra of DNA fragments. The analysis is effective in mapping the genome and marking genes for breeding valuable traits.
- PCR markers based on polymorphism analysis using PCR. For example, RAPD (Random Amplified Polymorphic DNA), PCR using a single short primer with a random nucleotide sequence; ISSR (Inter Simple Sequence Repeats), a specialized version of the RAPD method, in which the primer consists of amicrosatellite sequence; ISSR (Inter Simple Sequence Repeats), a specialized version of the RAPD method, in which the primer consists of a microsatellite sequence; AFLP (Amplified Fragment Length Polymorphism), a method combining RFLP and PCR; SSR (Simple Sequence Repeats), PCR with flanking primers to a short mini- or microsatellite repeat; IRAP (Inter Retrotransposone Amplified Polymorphism), analysis of polymorphic DNA regions amplified between retrotransposons; single nucleotide polymorphism SNP (Single Nucleotide Polymorphism), analysis of differences in a DNA sequence of one nucleotide in size in the genome or its fragment in representatives of the same species or between homologous regions ofhomologous chromosomes. To detect SNPs, hybridization methods are used, for example, detection using DNA chips; enzymatic methods (RFLP, TaqMan tests, capillary electrophoresis); methods based on the physical properties of DNA, for example, mass spectrometry, TGGE (Temperature Gradient Gel Electrophoresis), high-performance liquid chromatography under denaturing conditions; DNA sequencing, including modern high-throughput sequencing methods for mapping SNPs throughout the genome. The latter method is the most promising due to its high accuracy, reproducibility and automation of the process.
Biofertilizers for agricultural crops:
Biostimulants and multifunctional products
The use of modern agrobiotechnologies can significantly increase the efficiency of the agricultural sector and minimize its adverse impact on the environment. According to estimates of the interdepartmental working group for monitoring the implementation of biotechnologies under the Government of the Russian Federation, the total economic effect from the use of bio-preparations in crop production and livestock production in Russia can amount to more than 100 billion rubles per year with expenses worth 10.5 billion rubles [47].
A new direction in agricultural biotechnology is the development and use of biological products based on living organisms and (or) their metabolites that stimulate plant metabolism and induce their protective reactions, such as e.g. plant growth regulators. A multi-level system of induced phytoimmunity with numerous compounds participating in different stages of its manifestation makes it possible to study a large set of substances that can be used to control plant defense reactions and enhance resistance to phytopathogens. For example, the bio-preparation Bion activates the salicylic pathway with a signaling molecule of salicylic acid; biostimulator Novosil consists of triterpene acids isolated from green wood of Siberian fir; zircon P consists of hydroxycinnamic acids; Epin consists of brassinosteroids, Immunocytophyte is based on arachidonic acid, Ribav-Extra is based on L-alanine + L-glutamic acid, etc. In general, biological products that induce protective reactions are usually less effective compared to chemical drugs, and their use also requires certain conditions (especially temperature). At the same time, at low and medium levels of distribution and danger of phytopathogens, they are practically not inferior to chemical pesticides in terms of economic efficiency and even surpass them in safety [48].
One type of biofertilizers are bio-preparations containing bacteria that promote plant growth (Plant Growth-Promoting Bacteria, PGPB). When applied to seeds, plant surfaces or soil, they colonize the rhizosphere and promote growth by increasing the supply and availability of essential nutrients to the host plant [49]. PGPB fix molecular nitrogen, dissolve unavailable phosphorus and stimulate plant growth through the synthesis of specific substances, such as phytohormones, vitamins, siderophores, amino acids, enzymes, polyamines, free volatile fatty acids, biocidal compounds that inhibit the development of phytopathogens and pests. Inocula of such nitrogen fixers as Rhizobium, Azotobacter, Azospirillum and heterocytic cyanobacteria are used for cereals, legumes, and vegetable crops [50-52], and they turn out to be more effective than nitrogen fertilizers, about half of which are not absorbed by agricultural crops, but are released into the environment [53]. Other representatives of PGPB, the so-called phosphate-solubilizing bacteria, such as Pantoea agglomerans, Pseudomonas putida and many cyanobacteria [54], are capable of solubilizing insoluble phosphate from organic and inorganic phosphate sources, including phosphate fertilizers applied. The level of absorption of the latter by the plant does not reach even 20%, and a significant part of it is lost as a result of erosion and leaching [55], thus polluting groundwater and causing eutrophication of surface waters [56]. A wide range of microorganisms produce extracellular polymeric substances (EPS); they ate highly hydrated polymers consisting mainly of polysaccharides, proteins, and DNA. EPS are fundamental to microbial life and provide an ideal environment for chemical reactions, nutrient adsorption and protection from environmental stresses such as salinity and drought. Microbial EPS can enhance the aggregation of soil particles and benefit plants by maintaining environmental moisture and adsorbing nutrients [57]. Thus the plant growth-promoting bacteria are able to:
- Convert necessary biophilic elements from inaccessible or hard-to-reach forms into those available for absorption by plants, such as e.g. nitrogen, phosphorus, potassium, iron, zinc, etc. It was shown that PGPB inoculation can replace up to 30% of chemical nitrogen and phosphorus fertilizers, thus increasing crop yields by 20 -30% [58];
- Directly synthesize growth-stimulating substances (phytohormones, amino acids, vitamins, etc.);
- Produce EPS, on which the aggregation of soil particles, water-holding capacity of soils and adsorption of nutrients depend;
- Control the number of phytopathogens and pests due to biocidal activity;
- Improve soil health due to the ability to detoxify xenobiotics, for example, by decomposition of chemical pesticide residues.
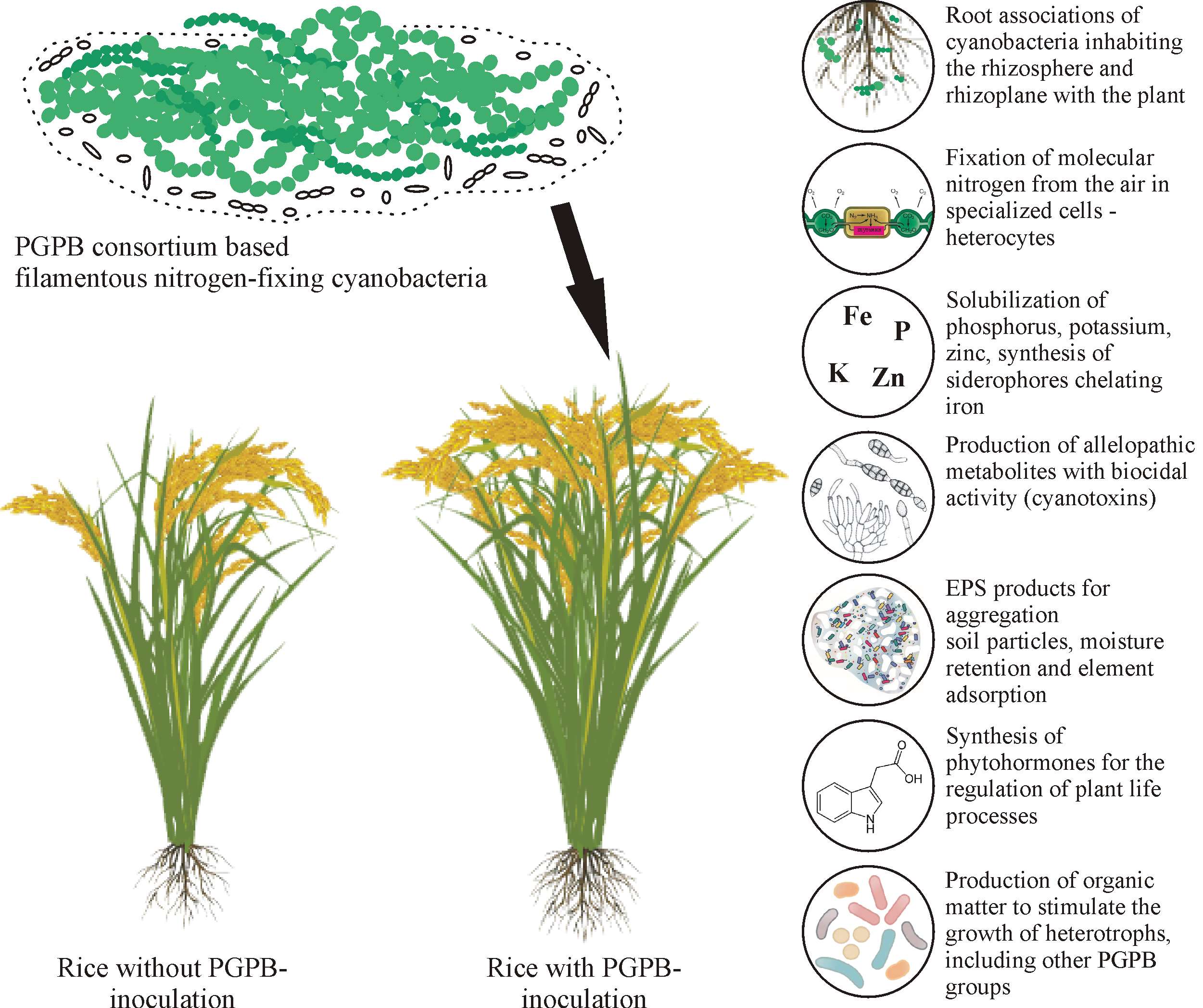
Figure 3. Mechanisms of rice growth promotion through the inoculation of multi-functional cyanobacteria-based drug.
Figure 3 provides a conceptual framework of the effects of PGPB on plant growth using rice and cyanobacteria as examples [50]. It is expected that in the future, accelerated commercialization and increased demand in the agricultural sector will be facilitated by new approaches to the development of multifunctional biological products, formulations with a longer shelf life (microencapsulation), search and/or creation ofeffective strains using genetic engineering methods [59].
Biological plant protection means
FAO estimates that up to 40% of the world's food crops are lost each year due to pests and plant diseases. Of the approximately 70 thousand adverse species, only 10% pose a serious danger; more than 2.5million tons of chemical pesticides are used annually to suppress them [48]. Chemical and biological plant protection means are not opposed or mutually exclusive in an integrated plant protection system. They interact to solve the task of increasing the productivity of agroecosystems and safety of agricultural products simultaneously reducing negative man-made impacts on the environment.
Today, the biological system of plant protection and plant nutrition provides scientifically based solutions that are not inferior in effectiveness to chemical ones, and in some aspects surpass them. Bio-pesticides are substances used to kill plant pests and pathogens, as well as various parasites and weeds; they are obtained mainly using microorganisms. In Russia, according to GOST R 56694-2015 “Renewable Sources of Raw Materials. Agricultural Resources. Terms and Definitions", bio-pesticides are biological plant protection means that are used to control pests of cultivated plants. They are either living objects orhighly active chemical compounds synthesized by living organisms. Bio-pesticides can be divided into three categories [60]:
- Bio-preparations based on microorganisms (bacteria, fungi, viruses, and protozoa) and their metabolic products.
- Bio-preparations from plants, plant extracts (pine needles, roses, barberry, ginseng, etc.) and other natural substrates, including GMO. For example, chitosan causes an increase in plant systemic resistance, which allows protection against diseases, pathogens, and pests [61]. Garlic oil has insecticidal properties [62].
- Pheromones are preparations based on natural compounds that do not have a toxic effect on harmful organisms, but only affect their behavior. They are usually used in the form of baits and traps for harmful insects.
The main driving forces of the world bio-pesticide market are the development of organic farming and more stringent environmental requirements for agricultural products.
Table 1. Examples of Russian bio-pesticides [66]
|
Active agent |
Phytopathogens/pests |
Commercial name of the bio-preparation |
|
CpGV codling moth granulosis virus |
Insects |
Carpovirusin, Madex Twin |
|
Bacterium Bacillus subtilis |
Fungi |
Alirin-B, Gamair, Fitolek |
|
Bacterium Bacillus thuringiensis |
Lepidoptera insects, spider mites and Colorado potato beetle larvae |
Lepidotsid, Bicol, Bitoxibacillin, Insetim |
|
Bacteria Proteus, Pseudomonas, Staphylococcus, Flavobacterium |
Nematodes of the Steinernematidae family |
Nemabakt, Anthonema-F |
|
Bacterium Sаlmonella enteritidis, var. Issatschenko |
Rodents |
Bactorodencide |
|
Bacterium Streptomyces avermitilis |
Root nematodes, insects |
Akarin, Fitoverm |
|
Fungi Arthrobotrys oligospora |
Nematodes |
Nematophaginous Mikopro |
|
Fungi Beauveria bassiana |
Insects |
Bioslip BV |
|
Fungi Paecilomyces fumosoroseus and Metarhizium anisopliаe subsp. atis |
Insects |
Pecilomycin, soil Cordyceps-Mikopro |
|
Fungi Trichoderma |
Fungi |
Trichocin, Trichoflor |
The benefits of using bio-pesticides include the following:
- Complete or partial refusal of chemical plant protection products, reduction of the overall pesticide load on agroecosystems and, as a result, improvement of the soil composition and increase of the soil fertility;
- Safety and high rate of biodegradation of bio-pesticides;
- Lower risk of the development of resistance in phytopathogens and pests;
- Selectivity of action against a wide range of harmful insects and phytopathogens;
- Application at any phase of the growing season and a short waiting period for harvesting after treatment;
- Use in integrated crop protection systems;
- High profitability due to the lower price of the preparation, its prolonged action and high efficiency when used correctly;
- The possibility of reorienting a number of farms to organic production.
The disadvantages are the preventative nature of action, where biopesticides must be applied before a crop disease has spread widely in a field and before insect pest populations become too numerous.
In Russia, the bio-pesticide market is at an early stage of development. Thus, currently in Russia only 9% of grain and 4% of sugar beet areas are treated with bio-pesticides [63]. According to the Union ofOrganic Farming, a biological plant protection system in the Russian Federation has been introduced into real practice on 2% of farmland [64]. Nevertheless, the bio-pesticide segment has doubled over the past 5years [65], and until 2030 its annual growth is projected to be 5-10%. The most popular is pre-sowing treatment of seeds and seeding material with bio-pesticides which inhibit infections and simultaneously protect seedlings from soil pathogenic microflora. Such treatment increases the germination rate and quality of plantings. The Table shows some preparations with biocidal activity produced in the Russian Federation. When registering biopesticides, manufacturing companies take into account their toxicity, pathogenicity, infectivity, ecotoxicology, environmental degradation and other indicators.
The lack of adequate scientific research on the toxin-producing capabilities of different groups ofmicroorganisms is the next obstacle to the accelerated production of new bio-pesticides and biological control agents. Other limiting factors for the development of the bio-pesticide market are the insufficient awareness and understanding of their benefits among agronomists, producers, and crop consultants. In addition, most agricultural enterprises are low-profitable and their long time experience in the use ofchemical plant protection means is dictated by commercial interests aimed at increasing the yield per unit of sown area, maintaining quality of the crop during storage and transportation. Issues of security and environmental friendliness often fade into the background. In Russia, the culture of farming in general and awareness of modern trends in agricultural practice are at a rather low level. Organic farming, which has become widespread in Europe, is just beginning to develop in this country.However, the low level of use ofbiopesticides in Russia does not reflect their important socio-economic role in society. Biological plant protection remains undervalued in situations where chemicals are ineffective, unavailable or prohibited. Examples include areas where crops for baby and dietary nutrition are grown, areas with a special environmental status, such as recreational, resort, forest, park, water protection zones and nature reserves, aswell as areas contaminated with heavy metals, organic substances, and radionuclides.
Test systems for diagnosing crop diseases
The successful development of plant cultivation is inextricably linked with crop protection against phytopathogen - pathogenic organisms that cause infectious plant diseases. The most known phytopathogens are:
- Viruses and viroids causing tobacco mosaic virus, raspberry bushy dwarf virus, potato spindle tuber viroid, etc.;
- Bacteria, including phytoplasmas, that cause crown gall, nightshade stolbur, scab, fire blight of fruit crops, etc.;
- Fungi and fungus-like organisms that cause late blight, anthracnose, powdery mildew, alternaria, smut, blister rust, etc.
In addition, plant diseases can be non-infectious in nature and be associated with abiotic environmental factors, for example, extreme conditions of temperature, humidity and light, deficiency orexcess of bioelements, etc.
Phytopathogens are characterized by the following properties:
- pathogenicity, i.e. the ability to cause disease;
- aggressiveness, i.e. the ability to intensively reproduce inside an infected plant;
- virulence, i.e. the ability of an infectious agent to cause disease or death of an organism.
Phytopathogens can be spread by vector pests, such as thrips carrying tomato bronzing virus; whiteflies that carry tomato yellow leaf curl virus; aphids carrying cucumber mosaic virus, etc. [67].
Annual crop losses caused by infectious diseases amount to at least 10-16% [68] and reach 80% when epiphytoties occur [69]. Undoubtedly, the effectiveness of combating plant infections is many times higher atthe first stages of their development, therefore timely, quick and accurate diagnosis of the disease is an important task of agricultural phytopathology. Identification of pathogens is also carried out during certification of seeds and plantings, phytosanitary monitoring of crops, selection and quarantine inspection ofimported seed material.
There are several main ways to identify phytopathogens:
- Morphological diagnosis, i.e. identification by external signs of the disease (symptoms), for example, growth retardation, changes in color, shape and size of various organs, necrotic lesions. Visual examination is the simplest and most traditional method, but also the least reliable: symptoms are universal and do not always appear quickly after infection.
- Testing on indicator plants - special indicator species that give a clear and typical response to infection with a specific pathogen, for example steppe pigweed, datura, garden quinoa, etc.
- The inclusion method based on the identification, using light or electron microscopy, of characteristic intracellular viral inclusions of amorphous and crystalline forms.
- Immune-enzyme analysis (IEA), consisting of two main stages: immune and enzymatic reactions. The immune reaction consists of the specific binding of an antigen characteristic of a given pathogen to adiagnostic antibody. Identification of the formed complex is carried out using an enzyme as a label for recording the signal (enzymatic reaction). One of the most common IEA variants is the ELISA test (Enzyme-Linked Immunosorbent Assay, ELISA). IEA-based methods are widely used to detect viruses, but much less frequently for the identification of fungi and bacteria due to the difficulty of obtaining antibodies with the required specificity. To quickly detect plant diseases in the field, immunostrips have been developed, such as e.g. ImmunoStrip® Tests (Agdia, USA).
- Molecular genetic diagnostics involves the accumulation of a large number of copies of the target pathogen DNA in a cultivated state or isolated from an affected plant. Varieties include direct polymerase chain reaction (PCR), reverse transcription PCR (for RNA viruses), inserted and real-time PCR (for quantification of target DNA), isothermal amplification (LAMP), DNA microarrays with ability to identify awide range of pathogens, digital PCR and high-throughput sequencing (NGS) technologies. The advantages of this group of methods are the specificity, sensitivity, and reliability of determining the phytosanitary state of agricultural plants. Currently, commercial diagnostic kits have been developed by domestic companies Synthol, Genbit, DNA Technology, etc.
Effective protection of crops from phytopathogens and their vectors should include not only early and effective diagnosis of the disease, but also a combination of biological, agrotechnical, chemical, physical and other methods aimed at preventing a complex of diseases, such as for example, a spatial isolation ofcultivated plants from sources of infection; the use of healthy seed material for planting; the destruction ofweeds that are reservoirs of infections; compliance with optimal timing, sowing norms and planting density; the use of chemical and biological agents to suppress phytopathogens and disease vectors, as well as the use of resistant plant varieties. Such a system for managing the phytosanitary state of agroecosystems is called integrated protection of plants and is designed to ensure their phytosanitary well-being [70].
Conclusion
Climate change, depletion of natural resources, population growth, and land degradation are putting additional pressure on agricultural food supplies. Currently, the relationship between the microbiomes of soil, plants, animals and humans is being actively discussed within the framework of the concept of “one health”: “healthy roots - healthy plants - healthy people” [71]. Soils are the cornerstone of health, they serve as a source and reservoir of both pathogens and beneficial microorganisms, and of the microbial diversity in general According to the UN FAO, 95% of food production is directly or indirectly linked to soil [72], while a third of soils worldwide have been degraded due to erosion, salinity, acidification, pollution and other negative impacts [73]. Maintaining a balance between the use of nature-like technologies and the latest achievements of bioengineering, i.e. partial abandonment of chemical pesticides and fertilizers in favor of biological products that stimulate plant growth and inhibit pests and phytopathogens, the development of genetically modified plant varieties and animal breeds, subject to their high productivity, safety and sustainability, the creation of test systems for diagnosing diseases of agricultural plants and animals, and as well as drugs for their treatment and prevention, we contribute to the necessary satisfaction of current human needs while preserving the environment and providing the rational use of resources without harm to future generations.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
Conflict of interest
The authors declare no conflict of interest.
References
- World Population Clock: 8.1 Billion People. Worldometers. Доступен по ссылке: https://www.worldometers.info/world-population
- United Nations. Department of Economic and Social Affairs. World Population Prospects (New York, 2022).
- A third of the world's food is wasted. The UN has launched a global campaign to combat food loss. Available at: https://news.un.org/ru/story/2019/10/1364302
- In world of wealth, 9 million people die every year from hunger, WFP Chief tells Food System Summit. Available at:: https://www.wfp.org/news/world-wealth-9-million-people-die-every-year-hunger-wfp-chief-tells-food-system-summit
- Agro-industrial complex of the future. A look at agriculture through the lens of big data analysis. Available at: https://www.agroinvestor.ru/analytics/article/31304-apk-budushchego
- Maxmen A. Crop pests: under attack. Nature, 501, 15–17 (2013). DOI: 10.1038/501S15a
- Inge-Vechtomov S.G. Genetics with the Basics of Selection (N-L, St. Petersburg, 2015).
- Kosolapov, V.M., Kozlov, N.N., and Klimenko, I.A. Genomic selection: stages of development. Bulletin of Russian Agricultural Science, 1, 8–12 (2018)
- Telem R.S., Wani S.H., Singh N.B., Nandini R., Sadhukhan R., Bhattacharya S., and Mandal N. Cisgenics - a sustainable approach for crop improvement. Genomics, 14(7), 468–476 (2013). DOI: 10.2174/13892029113146660013
- Maghari B.M. and Ardekani A.M. Genetically modified foods and social concerns. Avicenna J Med Biotechnol., 3(3), 109–117 (2011).
- Shevelukha V.S. et al. Agricultural biotechnology (Higher School, Moscow, 2003).
- Esvelt K.M. and Wang Н.Y. Genome-scale engineering for systems and synthetic biology. Molecular Systems Biology, 9, 641 (2013). DOI: 10.1038/msb.2012.66
- Mikhailova E.V., Khusnutdinov E.A., Chemeris A.V., and Kuluev B.R. Available arsenal of CRISPR/Cas systems for genome editing of plants. Plant Physiology, 69(1), 38–53 (2022). DOI: 10.31857/S0015330322010134
- Luo G., Najafi J., Correia P.M.P., Trinh M.D.L., Chapman E.A., Østerberg J.T., Thomsen H.C., Pedas P.R., Larson S., Gao C., Poland J., Knudsen S., DeHaan L., and Palmgren M. Accelerated domestication of new crops: yield is key. Plant Cell Physiol., 63(11), 1624–1640 (2022). DOI: 10.1093/pcp/pcac065
- Brookes G. and Barfoot P. GM crops: global socio-economic and environmental impacts 1996-2014. (PG Economics Ltd., Dorchester, 2016). DOI: 10.1080/21645698.2022.2118497
- Tilling T., Neeta L., Vikuolie M., and Rajib D. Genetically modified (GM) crops lifeline for livestock review. Agricultural Reviews, 31(4), 279–285 (2010).
- Klümper W. and Qaim M. A meta-analysis of the impacts of genetically modified crops. PLOS ONE, 9(11), e111629 (2014). DOI 10.1371/journal.pone.0111629
- Chen W., Chen L., Zhang X., Yang N., Guo J., Wang M., Ji S., Zhao X., Yin P., Cai L., Xu J., Zhang L., Han Y., Xiao Y., Xu G., Wang Y., Wang S., Wu S., Yang F., Jackson D., Cheng J., Chen S., Sun C., Qin F., Tian F., Fernie A.R., Li J., Yan J., and Yang X. Convergent selection of a WD40 protein that enhances grain yield in maize and rice. Science, 375(6587), eabg7985 (2022). DOI: 10.1126/science.abg7985
- Kovak E., Blaustein-Rejto D. and Qaim M. Genetically modified crops support climate change mitigation. Trends in Plant Science, 27(7), 627–629 (2022). DOI: 10.1016/j.tplants.2022.01.004
- Andersson H.C., Arpaia S., Bartsch D., Casacuberta J., Davies H., du Jardin P., Flachowsky G., Herman L., Jones H., Kärenlampi S., Kiss J., Kleter G., Kuiper H., Messéan A., Nielsen K.M., Perry J., Pöting A., Sweet J., Tebbe C., Johannes von Wright A., and Wal J.-M. Scientific opinion addressing the safety assessment of plants developed through cisgenesis and intragenesis. EFSA Journal, 10, 2561 (2012). DOI: 10.2903/j.efsa.2012.2561
- Nicolia A., Manzo A., Veronesi F., and Rosellini D. An overview of the last 10 years of genetically engineered crop safety research. Critical Reviews in Biotechnology, 34(1), 77–88 (2014). DOI: 10.3109/07388551.2013.823595
- Haslberger A.G. Codex guidelines for GM foods include the analysis of unintended effects. Nature Biotechnology, 21(7), 739–741 (2003). DOI:10.1038/nbt0703-739
- Clemente T.E. and Cahoon E.B. Soybean Oil: Genetic Approaches for Modification of Functionality and Total Content. Plant Physiology, 151(3), 1030–1040 (2009). DOI: 10.1104/pp.109.146282
- Nayar A. Grants aim to fight malnutrition. Nature, (2011). DOI: 10.1038/news.2011.233
- Li X., Wang Y., Chen S., Tian H., Fu D., Zhu B., Luo Y. and Zhu H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Plant Sci., 9, 559 (2018). DOI: 10.3389/fpls.2018.00559
- Ruiz-Lopez N., Haslam R.P., Napier J.A., and Sayanova O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. The Plant Journal, 77(2), 198–208 (2014). DOI:10.1111/tpj.12378
- Ueta R., Abe C., Watanabe T., Sugano S.S., Ishihara R., Ezura H., Osakabe Y. , and Osakabe K. Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci Rep, 7, 507 (2017). DOI: 10.1038/s41598-017-00501-4
- Sayre R., Beeching J.R., Cahoon E.B., Egesi C., Fauquet C., Fellman J., Fregene M., Gruissem W., Mallowa S., Manary M., Maziya-Dixon B., Mbanaso A., Schachtman D.P., Siritunga D., Taylor N., Vanderschuren H., and Zhang P. The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annual Review of Plant Biology, 62, 251–272 (2011). DOI:10.1146/annurev-arplant-042110-103751
- Meli V., Ghosh S., Prabha T., Chakraborty N., Chakraborty S., and Datta A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. PNAS, 107(6), 2413–2418 (2010). DOI: 10.1073/pnas.0909329107
- Kuzmina Yu.V. Genome editing methods to increase the shelf life of tomato fruits. Biotechnology and Plant Breeding, 3(1), 31–39 (2020). DOI: 10.30901/2658-6266-2020-1-o6
- Waltz E. Nonbrowning GM apple cleared for market. Nature biotechnology, 33(4), 326–327 (2015). DOI: 10.1038/nbt0415-326c
- Halterman D., Guenthner J., Collinge S., Butler N. , and Douches D. Biotech Potatoes in the 21st Century: 20 Years Since the First Biotech Potato. J. Potato Res., 93, 1–20 (2016). DOI: 10.1007/s12230-015-9485-1
- Kwon C.T., Heo J., Lemmon Z.H., Capua Y., Hutton S.F., Van Eck J., Park S.J., and Lippman Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Biotechnol., 38, 182–188 (2020). DOI: 10.1038/s41587-019-0361-2
- Banjara M., Zhu L., Shen G., Payton P. and Zhang H. Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol. Rep., 6, 59–67 (2012). DOI: 10.1007/s11816-011-0200-5
- Liang C. Genetically Modified Crops with Drought Tolerance: Achievements, Challenges, and Perspectives. In Drought Stress Tolerance in Plants, Eds by M. Hossain, S. Wani, S. Bhattacharjee, D. Burritt, L.S. Tran (Springer, Cham, 2016). pp. 531–547. DOI: 10.1007/978-3-319-32423-4_19
- Vaeck M., Reynaerts A., Höfte H., Jansens S., De Beuckeleer M., Dean C., Zabeau M., Van Montagu M. , and Leemans J. Transgenic plants protected from insect attack. Nature, 328, 33–37 (1987). DOI: 10.1038/328033a0
- Naranjo S. The Present and Future Role of Insect-Resistant Genetically Modified Cotton in IPMF (USDA. gov. United States department of agriculture, 2008). DOI: 10.1007/978-1-4020-8373-0_6
- Heck G.R., Armstrong C.L., Astwood J.D., Behr C.F., Bookout J.T., Brown S.M., Cavato T.A., DeBoer D.L., Deng M.Y., George C., Hillyard J.R., Hironaka C.M., Howe A.R., Jakse E.H., Ledesma B.E., Lee T.C., Lirette R.P., Mangano M.L., Mutz J.N., Qi Y., Rodriguez R.E., Sidhu S.R., Silvanovich A., Stoecker M.A., Yingling R.A., and You J. Development and Characterization of a CP4 EPSPS-Based, Glyphosate-Tolerant Corn Event. Crop Sci., 45(1), 329–339 (2005). DOI:10.2135/cropsci2005.0329
- Funke T., Han H., Healy-Fried M.L., Fischer M., and Schönbrunn E. Molecular basis for the herbicide resistance of Roundup Ready crops. PNAS, 103(35), 13010-13015 (2006). DOI: 10.1073/pnas.0603638103
- Widespread crop damage from dicamba herbicide fuels controversy. Available at: https://cen.acs.org/articles/95/i33/Widespread-crop-damage-dicamba-herbicide.html
- Kromdijk J., Głowacka K., Leonelli L., Gabilly S.T., Iwai M., Niyogi K.K., and Long S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science, 354(6314), 857–861 (2016). DOI:10.1126/science.aai887
- Evans J.R. Improving photosynthesis. Plant Physiology, 162(4), 1780–1793 (2013). DOI: 10.1104/pp.113.219006
- Karki S., Rizal G., and Quick W.P. Improvement of photosynthesis in rice (Oryza sativa L.) by inserting the C4 pathway. Rice, 6(1), 28 (2013). DOI: 10.1186/1939-8433-6-28
- Кosolapov V.M., Kozlov N.N., Klimenko I.A., and Zolotarev V.N. Genetic certification of achievements of forage crops breeding. Bulletin of Russian Agricultural Science, 5, 40–46 (2020). DOI: 10.30850/vrsn/2020/5/40-46
- Brickell C.D., Alexander C., Cubey J.J., David J.C., Hoffman M.H.A., Leslie A.C., Malécot V. , and Xiaobai J. International code of nomenclature for cultivated plants (Scripta Horticulturae, Leuven, 2016).
- Sukhareva A.S. and Kuluev B.R. DNA markers for genetic analysis of cultivated plant varieties. Biomics, 10(1), 69-84 (2018).
- Order of the Government of the Russian Federation dated July 4, 2023 N 1788-r “On approval of the Strategy for the development of production of organic products in the Russian Federation until 2030.” Available at: https://soz.bio/strategiyu-razvitiya-organicheskogo-pr/
- Zakharenko V.A. Biopesticides and plant protection products with non-biocidal activity in the integrated management of the phytosanitary state of grain agroecosystems. Agrochemistry, 6, 64-76 (2015).
- Kevin V.J. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255(2), 571–586 (2003). DOI:10.1023/A:1026037216893
- Alvarez A.L., Weyers S.L., Goemann H.M., Peyton B.M., and Gardner R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res., 54, 102200 (2021). DOI:10.1016/j.algal.2021.102200
- Song X., Bo Y., Feng Y., Tan Y., Zhou C., Yan X., Ruan R., Xu Q., and Cheng P. Potential applications for multifunctional microalgae in soil improvement. Environ. Sci., 10 (2022) DOI: 10.3389/fenvs.2022.1035332
- Solomon W., Mutum L., Janda T., and Molnár Z. Potential benefit of microalgae and their interaction with bacteria to sustainable crop production. Plant Growth Regul., 101, 53–65 (2023). DOI: 10.1007/s10725-023-01019-8
- Bouwman A.F., Beusen A.H.W., and Billen G. Human alteration of the global nitrogen and phosphorus soil balances for theperiod 1970–2050. Biogeochem. Cycles, 23, (2009). DOI: 10.1029/2009GB003576
- Rawat P., Das S., Shankhdhar D. and Shankhdhar S.C. Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. Soil Sci. Plant Nutr., 21, (2021). DOI: 10.1007/s42729-020-00342-7
- Elser J.J. Phosphorus: a limiting nutrient for humanity? Curr Opin Biotechnol., 23(6), 833–838 (2012). DOI: 10.1016/j.copbio.2012.03.001
- Alori E.T., Glick B.R., and Babalola O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front Microbiol., 8, 971 (2017). DOI: 10.3389/fmicb.2017.00971
- Costa O.Y.A., Raaijmakers J.M., and Kuramae E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front Microbiol. 9, 1636 (2018). DOI: 10.3389/fmicb.2018.01636
- Ghumare V., Rana M., Gavka O. ,and Khachi B. Bio-fertilizers-increasing soil fertility and crop productivity. Indust. Pollution Control., 30(2), 196–201 (2014).
- Aloo B.N., Tripathi V., Makumba B.A. ,and Mbega E.R. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Plant Sci., 13, 1002448 (2022). DOI: 10.3389/fpls.2022.1002448
- Khamidulina Kh.Kh. and Rabikova D.N. Green pesticides (advantages and challenges of implementation). Toxicological Bulletin, 3, 53–56 (2020). DOI: 10.36946/0869-7922-2020-3-53-56
- Benhamou N., Lafontaine P.J. and Nicole M. Induction of Systemic Resistance to Fusarium Crown and Root Rot in Tomato Plants by Seed Treatment with Chitosan. American Phytopathological Society, 84(12), 1432–1444 (2012). DOI: 10.1094/Phyto-84-1432
- Plata-Rueda A., Martínez L., Santos M., Fernandes F.L., Wilcken C.F., Soares M.A., Serrão J.E. and Zanuncio J.C. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci Rep., 7, 46406 (2017). DOI: 10.1038/srep46406
- Bio-protection is capturing minds and territories. Available at: https://www.agroxxi.ru/gazeta-zaschita-rastenii/zrast/biozaschita-zahvatyvaet-umy-i-territorii.html
- The level of implementation of agrobiotechnologies in Russia is only 2%. Available at: https://www.agroxxi.ru/zhurnal-agromir-xxi/stati-rastenievodstvo/uroven-vnedrenija-agrobiotehnologii-v-rossii-lish-2.html
- Skolkovo: in five years Russia will enter international markets with its own agrobiotechnologies.. Available at: https://old.sk.ru/news/b/press/archive/2017/02/09/skolkovo-cherez-pyat-let-rossiya-vyydet-na-mezhdunarodnye-rynki-s-sobstvennymi-agrobiotehnologiyami.aspx
- List of production means for use in the system of organic and biologized farming based on GOST 33080-2016 and international standards of organic agriculture. Available at: https://soz.bio/perechen-biopreparatov-i-bioudobren-2/
- Bogoutdinov D.Z., Fominykh T.S., Kastalyeva T.B., Girsova N.V., Pavlovskaya N.E., Gagarina I.N., Mishurov N.P., Nemenushchaya L.A., and Piskunova N.A. Methods for diagnosing pathogens of diseases of vegetable crops: analytical review (Rosinformagrotech, M., 2020).
- Chakraborty S. и Newton A.C. Climate change, plant diseases and food security: an overview. Plant Pathology, 60, 2–14 (2011). DOI: 10.1111/j.1365-3059.2010.02411.x
- Bebber D.P., Holmes T., Smith D. and Gurr S.J. Economic and physical determinants of the global distributions of crop pests and pathogens. New Phytologist, 202(3), 901-910 (2014). DOI: doi.org/10.1111/nph.12722
- GOST 21507-2013. Plant protection. Terms and Definitions. (Standardinform, M., 2014)
- Banerjee S. and van der Heijden M.G.A. Soil microbiomes and one health. Rev. Microbiol., 21, 6–20 (2023). DOI: 10.1038/s41579-022-00779-w
- Healthy soils are the basis for healthy food production. Available at:: https://openknowledge.fao.org/items/b56b85d3-9082-4512-8906-c31f2ff3f391
- Loss of nutrients in soils reduces the quality of fruits and vegetables. Available at: https://news.un.org/ru/story/2022/12/1435497
License
Copyright (c) 2024 Темралеева А. Д. (Автор)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.