Silicon mobilization in soils by Bacillus subtilis VKM B-1574
- Affiliations
-
- 1. National Research Tomsk State University
- 2. Skryabin Institute of Biochemistry and Physiology of Microorganisms RAS FRC PSCBR RAS
- Published:
- 2025-03-30
- Keywords:
- Soil microbiology; minerals destruction; silicon; phosphorus; Bacillus, Paenibacillus
Abstract
Silicon leaching plays an important role in the rock weathering, ores leaching, and soil-plant interactions. Mobile silicon compounds are vital for the growth of various plants. Meanwhile, the diversity of bacterial species and strains capable of actively silicon leaching from solid mineral material is strictly limited. The strain Bacillus sp. VKM В-1574 capable of solubilizing minerals in soils has been identified. This strain showed the highest similarity to the 16S rRNA gene sequence and the gyrB gene of Bacillus subtilis strain. Recently, this strain was shown to be able to leach silicon and other essential chemical elements from phosphorite ore, vermiculite, chernozem, and urban soils. The analysis of phosphorite bioleaching revealed a significant dissolution of silicon and Scanning electron microscopy confirmed the bacterial leaching of phosphorite rocks. Bioleaching of vermiculite was the highest in dissolved compounds compared to non-inoculated samples in case of numerous chemical elements including the rare ones. Application of B. subtilis VKM В-1574 to two soil samples on which wheat and cucumber were grown increased the amount of silicon compounds both in soil extracts and plants compared to non-inoculated variants. Some possible mechanisms of the silicon mobilization and the role of silicon in plants are discussed.
Full text
Introduction
Microorganisms play an important role in the transformation of various mineral compounds in soils [1]. For their growth, plants need silicon, potassium, phosphorus, and some other essential chemical elements which could be provided by soil bacteria able to solve mineral compounds [2-4]. This paper is dedicated to mobilization of mineral silicon (Si) that promotes the plants growth and brings about multiple beneficial effects [5, 6].
Many soil bacteria can degrade the Si-bearing minerals either by enzymatic degradation of crystals or their destruction yielding metabolic products (biogenic acids and alkali, chelating agents, etc.). The most known representative of active “silicate bacilli” is Paenibacillus mucilaginosus [7], formerly earlier known as Bacillus mucilaginosus [8]. It can synthesize silicase responsible for the destruction of Si-O bonds in the crystal lattice of clay minerals and those in the silicon-containing compounds [9-11]. Many attempts have been undertaken to apply the silicate bacilli for bioleaching valuable metals from silicate ores and bauxite [12, 13]. The use of bacilli to treat silicate minerals is limited for economic reasons, but this approach may be useful and feasible for the extraction of e.g. precious metals from technogenic slags.
The search for new species and strains capable of degrading silicate minerals continues worldwide. This interest is mainly related to the need to search for silicate-solubilizing bacteria to stimulate plant growth [14] and to obtain new sources of silicates for industrial purposes [15]. Special methods have been developed for screening silicolytic bacteria [16]. In recent decades, new silicate mineral-solubilizing strains have been reported, for example, isolated from the surface of weathered feldspar [17].
Our paper presents a study on the strain Bacillus sp. VKM В-1574 and its ability to degrade mineral compounds and release silicon and metals into the soils.
Materials and Methods
2.1 Microorganisms
The strain Bacillus sp. VKM B-1574 was deposited in the All-Russian Collection of Microorganisms (VKM) more than 70 years ago by the Leningrad Veterinary Institute under the name “Bacillus mucilaginosus” without specifying the source of its isolation. Accordingly, in our early publications we referred to this strain as “silicate bacteria” or “B. mucilaginosus” [18]. The strain showed high activity in the leaching of silicon- containing minerals [18]. In separate experiments on leaching the technogenic glassy (silicate) slags, we also used for comparison the following bacilli from the VKM: Paenibacillus alvei VKM B-502, P. edaphicus VKM B-2665, P. mucilaginosus VKM B-7519, and P. polymyxa VKM B-514.
2.2 Molecular methods of identification
DNA was isolated and purified according to the classic method [19]. Cells were suspended in 0.5 ml TE buffer (10 mM Tris; 1 mM EDTA, pH 8.0) and incubated with lysozyme (5 µg/ml) for 30 min at 37°C. Then 30 µl 10% SDS and 3 µl of 20 mg/ml proteinase K were added, the sample was mixed thoroughly and incubated for 1 h at 37°C. After addition of 100 µl of 5 M NaCl and 80 µl of CTAB/NaCl solution (10% CTAB, Hexadecyltrimethylammonium bromide, Sigma-Aldrich, in 0.7 M NaCl), the suspension was incubated for 10 min at 65°C. The DNA was purified with chloroform/isoamyl alcohol, precipitated with isopropanol, washed with 70% ethanol, dried and resuspended in 100 µl TE buffer.
PCR amplification was performed using a GeneAmp PCR System 2700 (“Applied Biosystems”, USA). The reaction mixture (25 µl) contained Taq polymerase buffer (Fermentas, Lithuania); DNA, 10 ng; dNTP (Fermentas, Lithuania), 0.2 mM; primers (Sintol, Russia), 0.1 µM of each; MgCl2, 2.5 mM; and Taq polymerase (Fermentas, Lithuania), 1 U. The 16S rRNA gene was amplified using the following pair of primers: 27f (forward: 5’-AGAGTTTGATCCTGGCTCAG-3’) and 1525r (reverse: 5’-AAGGAGGTGATCCAGCC-3’) under the following conditions: 95ºC for 5 min for denaturation of the target DNA; 30 cycles of denaturation at 94ºC for 30 sec, primer annealing at 55ºC for 30 sec and primer extension at 72ºC for 1 min 20 sec; 72ºC for 4 min for completion of amplification; cooling at 4ºC. The gyrB gene was amplified using the following pair of primers: UP-1 (forward: 5’-GAAGTCATCATGACCGTTCTGCAYGCNGGNGGNAARTTYGA-3’) and UP-2r (reverse: 5’-AGCAGGGTACGGATGTGCGAGCCRTCNACRTCNGCRTCNGTCAT-3’) [20] under the following conditions: 95ºC for 5 min for denaturation of the target DNA; 35 cycles of denaturation at 94ºC for 1 min, primer annealing at 60ºC for 1 min and primer extension at 72ºC for 2 min; 72ºC for 7 min for completion of amplification; and cooling at 4ºC. PCR-amplified DNAs were analyzed by gel electrophoresis on 1% agarose (low EEO, Sigma-Aldrich) for 40 min at 8.3 V/cm in TBE buffer (50 mM Tris-HCl, 48.5 mM boric acid, and 1 mM EDTA, pH 8.2). The amplified products were purified with GeneJET Gel Extraction Kit (Fermentas, Lithuania) according to the manufacturer’s instructions.
The DNA sequencing was carried out with the ABI PRISM® BigDye™ Terminator v. 3.1 reagent kit followed by the analysis of reaction products in an ABI PRISM 3730 Applied Biosystems automated DNA sequencing machine. The 16S rRNA and gyrase beta subunit (gyrB) gene sequencing was performed with universal primers 27f, 1525r and Up-1S (forward: 5’-GAAGTCATCATGACCGTTCTGCA-3’) [20].
The obtained 16S rRNA and gyrB genes sequences were compared with those deposited in GenBank using the BLAST (NCBI - http://www.ncbi.nlm.nih.gov). The sequences were aligned using the SILVA Incremental Aligner (SINA), version 1.2.11 software (https://www.arb-silva.de/aligner/) [21]. The similarity of sequences of the studied and type strains was calculated using the TaxonDC 1.2 [22]. A phylogenetic analysis was performed using EzTaxon-e (http://eztaxon-e.ezbiocloud.net/) [23] and MEGA version 7.0 software. The alignment of DNA gyrB sequences was performed using the Clustal Omega program (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic trees were constructed with the help of software MEGA version 7.0, using the neighbour- joining (NJ) [24], maximum-likelihood (ML) [25] minimum-evolution (ME) [26] methods. The maximum- likelihood tree constructed from the gyrB gene sequences of the strain VKM B-1574 and representatives of the closest species are shown in Figure 1. A bootstrap analysis based on 1000 resampling was used to evaluate the topology of the trees [25].
2.3 Leaching experiments
The leaching experiments were carried out with the following objects: i) phosphorite ore that contained both silicon and phosphorus, ii) Versoil - a mineral material formed by weathering of vermiculite, iii) chernozem soil, iii) polluted urban soil and technogenic glassy slags. The phosphorite ore was received from the Dzheroi Deposit, Uzbekistan, it had the following initial chemical composition (%): P2O5 - 13.6; CaO - 44.75; MgO - 1.05; CO2 - 20.88; Fe2O3 - 3.03; SiO2 - 2.7; Co - 0.11. The sterile ore samples were either exposed in the liquid medium under sterile conditions (blank) or inoculated with the bacterial strain VKM B-1574 in the same medium.
The medium composition was (g/l): NH4Cl - 1.2; MgSO4 × 7H2O - 0.1; KCl - 0.23; MnSO4 - 0.01; MgSO4 - 0.10; MnSO4 - 0.01; CoCl2 - 0.01; Na2HPO4 - 0.26; Na citrate - 1.20; glucose - 15; рН - 7.0. Both blank and experiment samples in flasks were incubated at 28ºC for one month. The results of leaching were evaluated by chemical analyses of liquid media and by electron microscopy of the rock surface. The goal of the experiment with phosphorite leaching was to study the phosphorus release by silicate bacilli.
The Versoil specimens were received from the Mining Institute, Kolsky Scientific Center of the Russian Academy of Sciences (RAS). This mineral is classified as a layered silicate of the following composition: Mg×(Mg,Fe)3 × [AlSi3O10]×(OH)2×4H2O. Vermiculite was leached according to the same scheme used in experiments with the phosphorite rock. Sterile versoil was placed in flasks with sterile nutrient medium (blank) or cultural liquid of the strain VKM B-1574 (experiment) at a ratio of 1 : 3 (solid phase : liquid medium). Both blank and experiment flasks were incubated at 28ºC for one month. The goal of the experiment with Aim of the Versoil / vermiculite leaching was to study the release of all inorganic compounds, including rare elements.
Сhernozem soil was sampled in the south of the Tomsk region, Siberia. It contained 8.4% of humus. The concentration of salts in the chernozem water extract made up (mg/100 g soil): NH4 - 14; NO3 - 15; P2O5 - 70; K2O - 80; pH was 5.8. The samples of urban soil were taken in the city of Tomsk suburbs; they represented a mixture of clay, various debris, and gray forest soil. Their humus content was only 0.5-0.9%; pH of its water extract was 4.2. The concentration of salts in the urban soil made up (mg/100 g): NH4 - 0; NO3 - 2.1; P2O5 - 5.9; K2O - 10.3. In the leaching experiments, the samples were moistened with distilled water up to 60% of their total moisture capacity. Then they were inoculated with Bacillus sp. VKM B-1574, 106 CFU/ml, 20 ml per 100 g soil (experiment) or supplemented with the equal volume of the nutrient medium (blank). The moistened soils were planted with wheat Triticum aestivum L. and cucumber Cucumis sativus L. Bacillus sp. VKM B-1574 was added additionally on the 7th and 15th days for wheat and on the 10th and 20th days for cucumbers.
In order to reveal possible mechanisms of bioleaching of technogenic silicate materials, we carried out a separate experiment at the G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms RAS, aimed at leaching technogenic glassy slags. The slags (green and black glass) were received from the Gvidon Gold Ltd., Russia. These slags were formed during the pyrometallurgical processing of secondary raw materials containing precious metals: silver, gold, palladium, platinum (60-200 g/ton), subjected to crushing and subsequent screening according to the size class <1 mm. The precious metals were in the form of small fused drops of a fine spherical fraction in the slags. These metals had no dissolved forms and their release from slags could be judged from the general destruction of the material or by release of iron and nickel as indirect indicators. The chemical composition of the slags according to the Gvidon Gold Ltd. made up (%): Fe- 51.00; S – 29,77; Si – 3.62; Ca – 1.75; Al – 1.21; Ni – 1.15; Mg – 0.86; Cu – 0.11; Co – 0.04. The leaching occurred due to exopolysaccharides produced by Bacillus sp. VKM B-1574 and other bacilli, namely: P. alvei VKM B-502, P. edaphicus VKM B-2665, P. mucilaginosus VKM B-7519, and P. polymyxa VKM B-514. To obtain exopolysaccharides, the grown bacterial biomass was collected, subjected to lysis, then centrifuged for 10 min at 13.4 thousand rpm to precipitate all possible mechanical impurities. The supernatant was then taken and 1 ml of the resulting bacterial slime was added to 9 ml of distilled water. The final solution was mixed with the ground slags at a ratio of 5 : 1 (liquid phase : solid phase). The exposure was carried out for 3 days at 28°C.
2.4 Chemical analyses
The concentration of mobile silicon compounds - monosilicon, polysilicon acids and organic silicon was determined photometrically at a wavelength of 660 nm [27, 28]. Each sample was placed in distilled water, 5 g/150 ml, and stirred with a blender. The procedure was repeated in 24 h, then the samples were centrifuged and the supernatants were analyzed for the presence of dissolved compounds. The concentration of monosilicon acids was estimated by a photometric method with ammonium heptamolybdate: the classic Mullen-Riley method [29] was used to eliminate the interfering effect of phosphates. Two aliquots of the obtained solution, 20 ml each, were sampled for the following analyses.
The first portion was ultrasonicated for 3 min to accelerate depolymerization of polysilicon acids. Then 0.1 g NaOH was added for the complete depolymerization of polysilicon acids and disintegration of water-soluble organosilicon compounds. The specimen was kept for 2 weeks, then the content of monosilicon compounds was estimated by the Mullen-Riley method [29]. The resulting value corresponded to the sum of monosilicon and polysilicon acids, and silicon of the mobile organosilicon compounds.
The second portion was supplemented with 0.5 g of activated carbon to adsorb organosilicon compounds. The specimen was then filtered, ultrasonicated and supplemented with 0.1 g NaOH (same as above). This resulting specimen was also saved for 2 weeks, then the concentration of silicon acids was estimated by the Mullen-Riley method [29]. The obtained value corresponded to the sum of monosilicon and polysilicon acids without silicon of any mobile organosilicon compounds.
The other dissolved inorganic compounds were analyzed by mass-spectrometry with inductively coupled plasma (ICP-MS, Perkin-Elmer ELAN-DRC-e) at the “Water” Center of the Tomsk Polytechnic University. All analyses were performed in accordance with the software package ELAN supplied with the equipment.
In experiments with the glassy slags, nickel and iron cations were analyzed using:
(i) Ion chromatograph Metrohm 838 for cation detection 844 UV/VIS Compact IC with the chromatographic column 6.1010.300 Metrosep (3×150 mm)
(ii) Atomic adsorption spectrophotometer SHIMADZU АА-6800 (Shimadzu, Japan) controlled by the WizAArd software.
2.5 Scanning electron microscopy of the phosphorite rock
The phosphorite powder was placed on the object table covered with a thin layer of glue, it was then then sprayed with silver under vacuum. The resulting sample was examined under the electron microscope JEM-100 CXII (JEOL, Japan) with the ASID-4D at the accelerating voltage of 20 kV.
2.6 Biochemical analyses
Biogenic organic products were analyzed in the liquid culturing medium after 48 h growth of Bacillus sp., VKM B-1574 at 29°C. The medium contained (g/l): NH4Cl - 1.2; MgSO4 × 7H2O - 0.1; KCl - 0.23; MnSO4 - 0.01; MgSO4 - 0.10; MnSO4 - 0.01; CoCl2 - 0.01; Na2HPO - 0.26; Na citrate - 1.20; glucose - 15; рН - 7.0. Phytohormones were analyzed using ELISA kits OLCHEMIM (Czech Republic). Ascorbic acid was determined by a reaction with 2.6-dichlorophenol-indophenol [30]; sugars - with potassium ferricyanide; the hydrolysis of polysaccharides was carried out in 2% HCl during 2 h [31]; the concentration of flavonoids was determined in alcohol extracts with AlCl3 [32]. Optical density was measured using Victor multifunction analyzer (Pribori Oy, Finland).
2.7 Statistics
All experiments and analyses were carried out in 3 replicates. The statistical analysis was performed using standard methods provided by the Excel program. The analysis of variance (ANOVA) was used to determine the validity of the data obtained. All the data presented in the article are reliable (p > 99%).
Results
3.1 Identification of Bacillus VKM B-1574
The 16S rRNA gene sequence for Bacillus sp. VKM B-1574 contained 1468 bp nucleotides. EzBioCloud analysis showed that the strain VKM B-1574 had the highest sequence similarity (100%) to the 16S rRNA gene sequence of the type strain of the species, namely: B. subtilis subsp. subtilis NCIB 3610T. The 16S rRNA gene sequence similarities to type strains of all the other species of B. subtilis were below 99.79%.
In the whole, species of the B. subtilis group have a high phenotypic similarity and identical or almost identical 16S rRNA gene sequences; they however differ in the fatty acid composition and have low DNA-DNA hybridization values among species [33]. For B. subtilis, a high correlation between sequence similarity values of the gyrase beta-subunit (gyrB) and DNA-DNA linkage has already been shown [33, 34]. Thus, the gyrB gene sequence-based phylogenetic analysis can be used to distinguish species of this group at the inter- and intra- species level [35]. We analyzed the gyrB gene analysis of the strain VKM B-1574. The obtained gyrB sequence was 771 nt in length. Pairwise comparisons of gyrB gene sequences showed that the closest relative (99.9% sequence similarity) of the strain VKM B-1574 was B. subtilis BCRC 10255T = NCIB 3610T. The phylogenetic trees calculated by ML, MJ and ME methods clearly demonstrated a similar topology and indicated that strain VKM B-1574 is placed in the monophyletic cluster of the B. subtilis-group and forms a coherent cluster with the type strain B. subtilis BCRC 10255T (the tree is shown in Fig. 1).
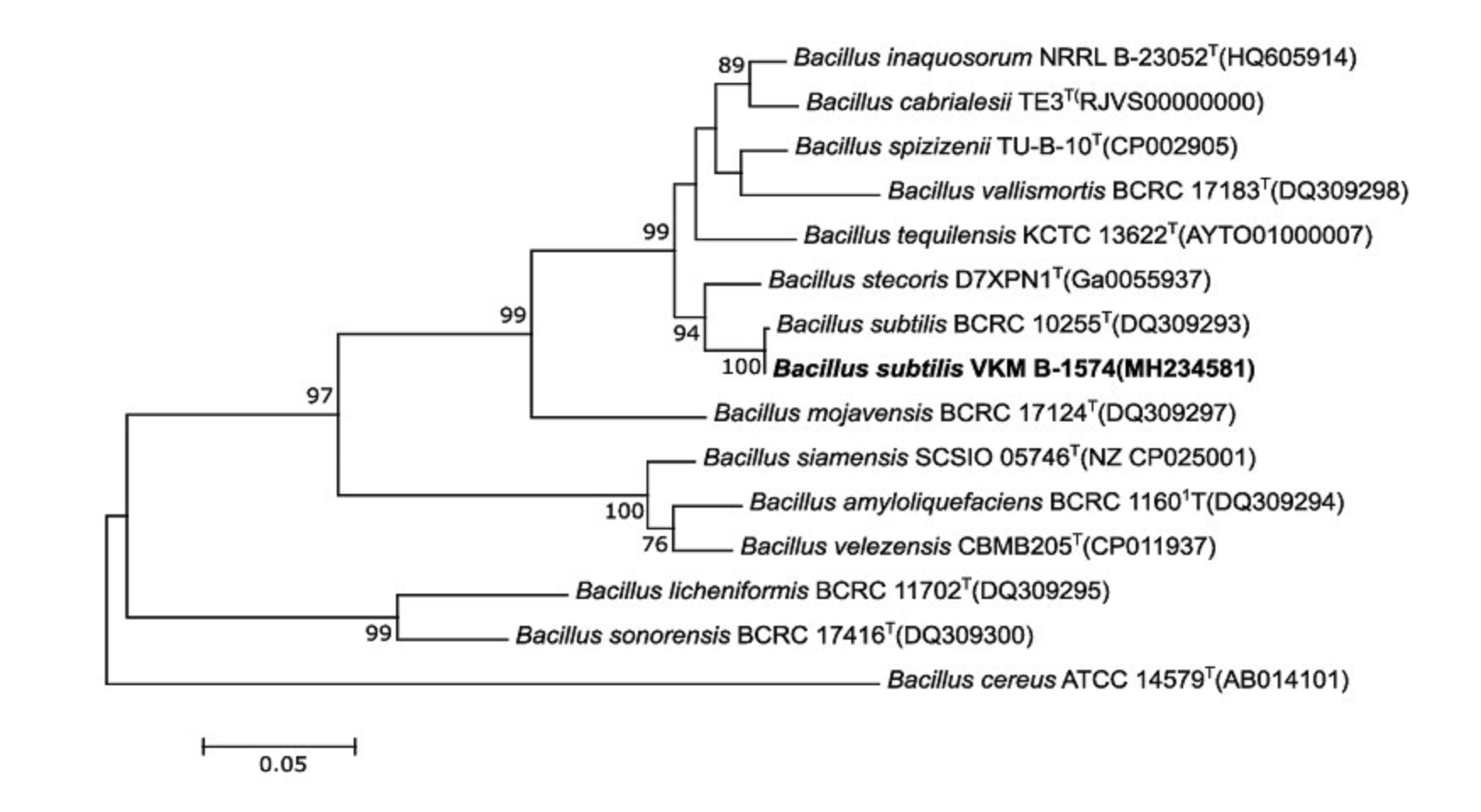
Figure 1. Maximum-likelihood phylogenetic tree based on partial gyrB gene sequences, showing the position of strain VKM B-1574 among the closest species of the genus Bacillus. Numbers at nodes are bootstrap values shown as percentages of 1000 replicates; only values >50% are shown. GenBank accession numbers are given in parentheses. Bar is 0.05 substitutions per a nucleotide.
3.2 Si and P leaching from the phosphorite ore
The results of phosphorite leaching are shown in Table 1 and Fig.2. The bioleaching process was confirmed by scanning electron microscopy which revealed the presence of multiple cells on the rock surface and also certain changes in the rock structure.
Table 1. Si and P leaching from phosphorite ore by B. subtilis VKM B-1574.
|
Incubation, days |
Number of bacteria in the inoculated experiments, 108 cells/ml |
Dissolved Si, mg SiO2 /l |
Dissolved P, mg (РО4)2- /l |
||
|
Experiments with B. subtilis VKM B-1574 |
Sterile (blank) variants |
Experiments with B. subtilis VKM B-1574 |
Sterile (blank) variants |
||
|
5 |
0.0026 |
9.25 |
6.25 |
0.55 |
0.45 |
|
10 |
11.5 |
16.75 |
6.88 |
1.25 |
0.63 |
|
30 |
22.0 |
17.25 |
7.10 |
1.63 |
0.72 |
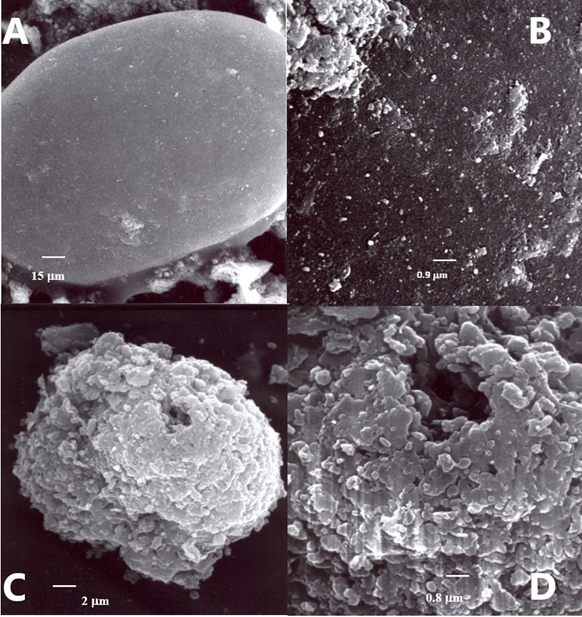
Figure 2. Scanning electron microscopy of the surface of phosphorite rocks after 30 days exposure in the liquid medium. А, B – blank, without B. subtilis VKM B-1574; C, D – experiment with B. subtilis VKM B-1574. The colonization of the mineral by bacterial cells is evident.
3.3 Vermiculite leaching
In the experiments with vermiculite, the ability of B. subtilis VKM B-1574 to leach various chemical elements including the rare earth ones was studied. The chemical analyses of dissolved salts after 30 days exposition revealed a high activity of bacteria in the leaching process, it was confirmed by the presence of high concentrations of dissolved chemical compounds in the inoculated samples compared to sterile (blank) ones: the concentration of dissolved iron increased 22.6-fold, magnesium increased 4.6-fold, silicon – 3.7-fold, etc. (Table 2). It is noteworthy that the concentration of dissolved P, Se, B, and some rare earth elements decreased probably due to their selective absorption by bacteria.
Table 2. Vermiculite leaching in the liquid medium, mg/l.
|
Chemical element |
Sterile (blank) leaching |
Leaching with B. subtilis VKM B-1574 |
Chemical element |
Sterle (blank) leaching |
Leaching with B. subtilis VKM B-1574 |
|
Na |
484.47 |
519.03 |
Ni |
0.037 |
0.17 |
|
K |
128.56 |
133.53 |
Rb |
0.032 |
0.012 |
|
Ca |
122.72 |
238.31 |
Li |
0.020 |
0.036 |
|
Mg |
84.67 |
387.71 |
Mo |
0.020 |
0.031 |
|
Si |
26.65 |
98.02 |
Co |
0.015 |
0.056 |
|
P |
2.32 |
0.72 |
Ge |
0.010 |
0.015 |
|
Fe |
1.61 |
36.43 |
Sc |
<0.01 |
<0.01 |
|
Sr |
0.79 |
1.21 |
Ga |
0.009 |
0.0090 |
|
B |
0.42 |
0.30 |
Se |
0.0088 |
0.0026 |
|
Cu |
0.12 |
0.17 |
Zr |
0.0032 |
0.019 |
|
Mn |
0.085 |
3.41 |
Nb |
0.0029 |
0.024 |
|
Al |
0.078 |
25.27 |
Y |
0.00012 |
0.0028 |
|
Ti |
0.076 |
1.48 |
Ag |
0.000061 |
0.00043 |
|
Zn |
0.061 |
0.39 |
V |
<0.000005 |
<0.000005 |
|
Cr |
0.054 |
0.25 |
Be |
<0.000004 |
0.00071 |
3.4 Formation of mobile Si-compounds in the soils
To study a possible positive role of B. subtilis VKM B-1574 in soil improvement, we analyzed the final concentrations of mobilized silicon, as monosilicic and polysilicic acids, and organosilicon compounds both in soils and plants. It is evident that organosilicon compounds were present in plants not soil. The concentration of monosilicic acids was higher both in chernozem and urban soil. In the whole, the concentration of mobilized Si-compounds was higher in the inoculated samples compared to the blank (non-inoculated) ones (Tables 3, 4).
Table 3. Silicon compounds in the chernozem and cucumber plants, mg SiО2 /100 g, in 60 days.
|
|
Soil, blank |
Soil, inoculated |
Cucumber plant, blank |
Cucumber plant, inoculated |
|
Monosilicic acids |
23.0 |
29.0 |
71.8 |
84.8 |
|
Polysilicic acids |
13.7 |
9.2 |
30.4 |
38.6 |
|
Organosilicon compounds |
<1.0 |
<1.0 |
6.2 |
17.0 |
|
Total |
36.7 |
38.2 |
108.4 |
140.4 |
Table 4. Silicon compounds in the urban soil and wheat plants, mg SiО2 /100 g, in 15 days.
|
|
Soil, blank |
Soil, inoculated |
Wheat plant, blank |
Wheat plant, inoculated |
|
Monosilicic acids |
5.7 |
12.1 |
32.0 |
30.6 |
|
Polysilicic acids |
2.6 |
7.0 |
7.6 |
29.1 |
|
Organosilicon compounds |
<1.0 |
<1.0 |
33.3 |
39.5 |
|
Total |
8.3 |
19.1 |
72.9 |
99.2 |
3.5 Active leaching compounds
The ability of many bacilli species to remove biogenic elements from minerals and soil is well known. In attempts to understand the mechanism of leaching, it was supposed that bacilli mainly release silicon and phosphorous with biogenic organic acids [36-38]. Moreover, some investigators showed that B. mucilaginosus degraded mineral silicon compounds with polysaccharides but suggested that polysaccharides simply adsorbed these biogenic organic acids [10]. Later, we showed that polysaccharides themselves could destroy polysilicic mineral structures [11]. This finding is in agreement with a previously proposed chemical mechanism [39]. In good agreement with these data, we performed a chemical analysis of the culture medium after 48 h growth of B. subtilis VKM B-1574, which showed the presence of a significant amount of both polysaccharides and biogenic organic acids, including ascorbic and keto-acids (Table 5). Thus, B. subtilis VKM B-1574 produces slime and acids and can affect minerals in two ways: either by destroying crystal lattices with enzymes or by breaking them down with microbial slime. In addition, we showed that during cultivation B. subtilis VKM B-1574 accumulated various biologically active substances in the liquid medium (Table 5).
It can be assumed that the bacterial leaching activity and mechanisms of its realization depend not only on the selected strain/species, but also on the characteristics of the processed mineral raw material. The possibility of using B. subtilis BKM B-1574 for industrial extraction of metals from glassy slags as secondary mineral raw materials was a separate area of research. The main goal of these studies was the recovery of precious metals, however, since they were not dissolved, the calculated indicators of mineral raw material degradation were the extraction of Ni and Fe [11, 40].
Table 5. Content of some biogenic compounds in B. subtilis VKM B-1574 culture medium after the 48 h growth.
|
Biogenic compounds |
Concentration, mg/l |
Biogenic compounds |
Concentration, mg/l |
|
Ascorbic acid |
1875 |
Mixed ketoacids |
17.25 |
|
Total sugars |
316 |
Pyruvic acid |
0.98 |
|
Polysaccharides |
266 |
Ketoglutaric acid |
0.32 |
|
Mixed flavonoids |
163 |
Dihydrozeatin riboside |
0.037 |
|
Reducing sugars |
50 |
Isopentenyl adenosine |
0.005 |
|
Indole-3-acetic acid |
25.9 |
Transzeatin riboside |
0.0004 |
Table 6. Comparison of the Fe- and Ni-leaching from technogenic slags with exopolysaccharides of different bacilli (according to [11]).
|
Variant of inoculation |
Concentration in the medium, mg/l |
|
|
Ni |
Fe |
|
|
- (blank) |
0.08 |
6.82 |
|
Bacillus sp. VKM B-1574 |
<0.05 |
4.00 |
|
Paenibacillus alvei VKM B-502 |
<0.05 |
8.00 |
|
P. polymyxa VKM B-514 |
< 0.05 |
8.00 |
|
P. edaphicus VKM B-2665 |
0.78 |
134.82 |
|
P. mucilaginosus VKM B-7519 |
0.86 |
143.45 |
We compared B. subtilis VKM B-1574 with other bacilli for slag degradation and the results are presented in Table 6. It can be seen that the exopolysaccharides of only two cultures used had the required industrial leaching activity, namely from P. edaphicus VKM B-2665 and P. mucilaginosus VKM B-7519. Exopolysaccharides from other bacilli, including B. subtilis VKM B-1574, were not efficient enough to transfer these metals into solution. Thus, the data obtained may provide evidence that the degradation of glassy silica bonds of B. subtilis VKM B-1574 is provided by bacterial enzymes rather than exopolysaccharides.
Comparing this information with the reported data on active metabolites of B. subtilis VKM B-1574 (Table 5) and silicon mobilization from other sources (Tables 2-4) opens a wide field for future studies.
In general, the results of scanning electron microscopy (Fig. 2) and analysis of the content of some biogenic compounds (Table 5) showed that B. subtilis strain VKM B-1574 can be used together with minerals, forming complex fertilizers.
Discussion
The stimulating effect of Si on plants is widely known and used in practice: silicon fertilizers are used in agriculture to increase the yield of cultivated plants [41]. The application of Si+P fertilizers enhances the activity of soil bacteria to mobilize essential chemical elements from solid minerals. In particular, it is a proven way to support plants with available phosphorus compounds [42].
Our experiments with vermiculite have shown that bacteria attack aluminosilicates; this leaching results in the dissolution/mobility of numerous chemical elements, including silicon and aluminum. Divalent cations are recovered to the greatest extent compared to passive chemical leaching. To date, a large amount of information has been accumulated on the ability of bacilli to degrade various minerals [9-11, 43]. According to published data and our results, biogenic soluble compounds include organic acids (gallic, oxalic, citric, salicylic, pyrocatecholic, benzoic, etc.), exopolysaccharides, and some enzymes (hydrolases, oxidoreductases, etc.). As shown in Table 5, biochemical analyses of the culture medium after 48 hours of growth of B. subtilis VKM B-1574 revealed the presence of significant amounts of not only polysaccharides, sugars and acids, but also important bioactive compounds. Thus, bacteria can (i) degrade minerals by acids, enzymes or polysaccharides, and (ii) stimulate plant growth by other mechanisms. B. subtilis VKM B-1574 appears to possess both abilities, which is important for the practical use of these microorganisms in agriculture.
It has been shown that in the process of cultivation B. subtilis can accumulate various biologically active substances in the medium, in addition to those listed in Table 5. These bacteria are able to accumulate about 200 antibiotics, amino acids, vitamins, phytohormones, as well as carboxylic organic acids (lactic, acetic, and butyric acids), di- and tricarboxylic acids - oxalic, malic, and citric acids [44]. We found three types of cytokinins: dihydrozeatin riboside, isopentenyladenosine, and transzeatinriboside. After inoculation of cucumber plants with this bacterial strain, the concentration of cytokinins and indole-3-acetic acid increased, thus stimulating plant growth. We also found that treatment of wheat with B. subtilis VKM B-1574 promoted significant strengthening of cereal stem and leaves due to the accumulation of silicon in plant tissues [18]. The ability of strain VKM B-1574 to synthesize flavonoids is also presented in Table 5. These compounds are phenolic in nature. For another B. subtilis strain [44], it has already been shown that these bacteria produce phenylacetic acid (29.03 %) and 4-hydroxyphenylacetic acid (10.49 %) in significant amounts, and these compounds are able to induce root formation in plants.
In the presented work, we demonstrated the ability of B. subtilis VKM B-1574 to mobilize silicon and incorporate it into biogenic silicon-containing compounds. Biogenic leaching of other inorganic salts from solid minerals was also demonstrated. In general, these results suggest that the application of B. subtilis VKM B-1574 to different soils may have a positive effect on the process of mobilization of major chemical elements into plant-available form.
Organosilicon compounds were not detected in the studied soils (Table 4), but their content increased in plants after bacteria application. The results of the experiments showed that the content of biolytes in wheat and cucumber plants was higher than in the blank variant. In plants grown on chernozem, an increase in the content of organosilicon compounds more than 2-fold was observed; in urban soil, the level of these compounds increased, but not so significantly. Apparently, polysilicic acids serve as a basis for the formation of plant biolytes.
Water respiration and Si polymerization are the two main factors in obtaining high silicic acid concentration in plant shoots [45]. The intensity of Si uptake also varies depending on the plant species [46]. In cereals and cucumber, Si transport can be mediated by different specific systems and requires different energy fluxes. In wheat, additional Si in the form of monomeric silicic acid is present in the xylem [46]. Cucumber plants do not absorb Si as rapidly as wheat plants, but when soil Si concentrations are high, Si uptake by the cucumber plant can increase and the Si content of the plant leaves can be as high as in cereals [47].
Conclusions
Previously, we used the strain of Bacillus sp. VKM B-1574 for leaching and mobilization of major nutrients from minerals in order to use them in the soil-plant system [48]. In the presented work, we identified Bacillus sp. VKM B-1574 as B. subtilis with a very high similarity to the type strain of the species both in terms of 16S rRNA gene sequence and gyrB genes. We also showed its high efficiency in leaching phosphate ore and vermiculite (Versoil), which in turn can be used as soil fertilizer. In addition, we inoculated B. subtilis VKM B-1574 into two types of soils seeded with wheat and cucumber and demonstrated an increase in the amount of mobilized Si compounds in both soil extracts and plant tissues compared to uninoculated (blank) variants.
Some possible mechanisms of Si mobilization and the role of silicon in plants are discussed. Various bacterial metabolites - acids, sugars, exopolysaccharides, enzymes – of the strains can be used in the mobilization of silicon in a mineral-dependent manner. We did not find active destruction of glass by biogenic exopolysaccharides; nevertheless, based on the study we can state that B. subtilis strain BKM B-1574 actively leaches silicon-containing minerals and can be used together with minerals to create complex fertilizers. The fact that the studied active strain belongs to B. subtilis allows expanding the search for new industrial strains of this species.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
References
- Torsvik V. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 1, 240-245 (2002). DOI: 1016/s1369-5274(02)00324-7.
- Basak B., Sarkar B., Biswas D.R., Sarkar S., Sanderson P. and Naidu R. Bio-intervention of naturally occurring silicate minerals for alternative source of potassium: challenges and opportunities. Adv. Agron., 141, 115-145 (2017). DOI: 10.1016/bs.agron.2016.10.016.
- Greger , Landberg T. and Vaculík M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants., 7 (2), 41 (2018). DOI: 10.3390/plants7020041.
- Pastore , Kernchen S. and Spohn M. Microbial solubilization of silicon and phosphorus from bedrock in relation to abundance of phosphorus-solubilizing bacteria in temperate forest soils. Soil Biol. Biochem., 151, 108050 (2020). DOI: 10.1016/j.soilbio.2020.108050.
- Barão , Teixeira, R., Vandevenne F. and Struyf E. Silicon mobilization in soils: the broader impact of land use. Silicon., 12, 1529–1538 (2020). DOI: 10.1007/s12633-019-00245-y.
- Katz O., Puppe D., Kaczorek D., Prakash N.B. and Schaller, J. Silicon in the soil–plant continuum: intricate feedback mechanisms within Plants., 10, 652 (2021). DOI: 10.3390/plants10040652.
- Hu X.F., Li S.X., Wu J.G., Wang J.F., Fang Q.L. and Chen J.S. Transfer of Bacillus mucilaginosus and Bacillus edaphicus to the genus Paenibacillus as Paenibacillus mucilaginosus. Int. J. Syst. Evol. Microbiol., 60, 8-14 (2010). DOI: 1099/ijs.0.008532-0.
- Avakyan Z.A., Pivovarova T.A., Karavaiko G.I. Bacillus mucilaginosus sp. nov. In Validation of the publication of new names and new combinations previously effectively published outside the IJSB, List no. 66. Int. J. Syst. Bacteriol., 48, 631–632 (1988).
- Karavaiko G.I. Microbial destruction of silicate minerals. Trudy Instituta mikrobiologii imeni S.N. Vinogradskogo RAN [Proceedings of the Vinogradskii Institute of Microbiology, Ac. Sci.], 172- 196 (2004).
- Liu , Xu X., Wu X., Yang Q., Luo Y. and Christie P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health., 28 (1-2), 133-40 (2006). DOI: 10.1007/ s10653-005-9022-0.
- Abashina T. N., Delegan Y. A., Yatskiv A. A., Vainshtein M. B., and Kumar S. Bioleaching metals from glassy slags with silicate bacteria. In Proc. the 5th Pushchino school-conference “Biochemistry, Physiology and Biospheric Role of ” (Pushchino, Publishing house “Water: Chemistry and Ecology”, 2018), pp. 42-44.
- Sukla B., Panchanadikar V.V., Kar R.N. Microbial leaching of lateritic nickel ore. World J Microbiol. Biotechnol., 9, 255–257 (1993). DOI: 10.1007/BF00327850.
- Zhan Liu J., Chen Y. and Sun D. Single and coorperative bauxite bioleaching by silicate bacteria. IERI Procedia., 5, 172-177 (2013). DOI: 10.1016/j.ieri.2013.11.088].
- Cruz J.A., Tubana B.S., Fultz L.M., Dalen M.S. and Ham, J.H. Identification and profiling of silicate- solubilizing bacteria for plant growth-promoting traits and rhizosphere competence. Rhizosphere., 23, 100566 (2022). DOI: 1016/j.rhisph.2022.100566.
- Toender E., and Borchert M. Use of enzymes having silicase activity. Patent USA № US8822188B2, publ. 02.09.2014.
- Kaur , Sharma A. Bhardwaj N.K., Singh A., Dalal S. and Sharma J. A novel, simple, and quick plate assay to screen silicolytic bacteria and silicase production using different substrates. Biores. Technol. Rep., 17, 100971 (2022). DOI: 10.1016/j.biteb.2022.100971.
- Sheng X.F., Zhao F., He L.Y., Qiu G. and Chen L. Isolation and characterization of silicate mineral- solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol., 54 (12), 1064-1068 (2008). DOI: 1139/W08-089.
- Sokolova M., Belogolova G., Akimova G. and Vayshlya O. The role of silicate rhizobacteria in the biosorption of silicon in the soil-plant system during polyelemental soil contamination. Agrokhimiya., 1, 71-77 (2019).
- Ausubel F.M., Brent M., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A. and Struhl, K. Current Protocols in Molecular Biology. New York: Wiley., 650 (1994).
- Yamamoto and Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol., 61, 1104-1109 (1995). DOI: 10.1128/aem.61.3.1104-1109.1995.
- Pruesse , Peplies J. and Glöckner F.O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics., 18, 1823-1829 (2012). DOI: 10.1093/bioinformatics/bts252.
- Tarlachkov V. and Starodumova I.P. Taxon DC Calculating the similarity value of the 16S rRNA gene sequences of prokaryotes or ITS regions of fungi. J. Bioinf. Genomics., 16, 1-4 (2017). DOI: 10.18454/ jbg.2017.3.5.1
- Yoon , Ha S., Kwon S., Lim J. and Kim Y. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol., 67( 5), 1613-1617 (2017). DOI: 10.1099/ijsem.0.001755.
- Saitou N. and Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Biol. Evol., 4, 406-425 (1987). DOI: 10.1093/oxfordjournals.molbev.a040454.
- Felsenstein Confidence limits on phylogenies: An approach using the bootstrap. Evolution., 39, 783- 791 (1985). DOI: 10.1111/j.1558-5646.1985.tb00420.x.
- Takahashi and Nei M. Efficiencies of fast algorithms of phylogenetic inference under the criteria of maximum parsimony, minimum evolution, and maximum likelihood when a large number of sequences are used. Mol. Biol. Evol., 17, 1251-1258 (2000). DOI: 10.1093/oxfordjournals.molbev.a026408.
- Matychenkov, V.V. and Shnaider G.S. Mobile silicon compounds in some South Florida soils. Pochvovedenie., 12, 1448-1453 (1996).
- Matichencov V. V., and Bocharnikova E. A. The relationship between silicon and soil physical and chemical properties. In Silicon in Agriculture, Studies in Plant Science, vol. 8, Ed. by Datnoff L. E., Snyder G. H., and Korndörfer G. H. (Amsterdam, The Netherlands, Elsevier, 2001), pp. 209–219.
- Mullin J., and Riley J.P. The colorimetric determination of silicate with special reference to sea and natural Anal. Chim. Acta., 12, 162-176 (1955). DOI: 10.1016/S0003-2670(00)87825-3.
- Hughes E. Titrimetric determination of ascorbic acid with 2,6-dichlorophenol indophenol in commercial liquid diets. J. Pharm. Sci., 72 (2), 126-129 (1983). DOI: 10.1002/jps.2600720208.
- Liu and Jiang G. Spectrophotometric flow injection determination of total reducing sugars in tobacco based on oxidation by ferricyanide and formation of Prussian blue. Analytical Letters., 34 (11), 1923-1934 (2001). DOI: 10.1081/AL-100106122.
- Mahboubi M., Kazempour N. and Boland Nazar A.R. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of Scrophularia striata Boiss extracts. Jundishapur J. Nat. Pharm. Prod., 8 (1), 15-19 (2013). PMCID:
- Rooney P., Price N.P., Ehrhardt C., Swezey J.L. and Bannan J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis sp. in aquosorum subsp. nov. Int. J. Syst. Evol. Microbiol., 45, 2420-2436 (2009). DOI: 10.1099/ijs.0.009126-0.
- Kasai , Watanabe K., Gasteiger E., Bairoch A., Isono K., Yamamoto S., and Harayama S. Construction of the gyrB database for the identification and classification of bacteria. In Genome Informatics (Tokyo, Universal Academic Press, 1998), vol. 21.
- Wang L.T., Lee F.L., Tai C.J. and Kasai H. Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA-DNA hybridization in the Bacillus subtilis Int. J. Syst. Evol. Microbiol., 8, 1848-1850 (2007). DOI: 10.1099/ijs.0.64685-0.
- Ameen F., AlYahya S.A., AlNadhari S., Alasmari H., Alhoshani F. and Wainwright M. Phosphate solubilizing bacteria and fungi in desert soils: species, limitations and mechanisms. Arch. Agron. Soil , 65 (10), 1446-1459 (2019). DOI: 10.1080/03650340.2019.
- Bist V., Niranjan A., Ranjan M., Lehri A., Seem K. and Srivastava S. Silicon-solubilizing media and its implication for characterization of bacteria to mitigate biotic Front. Plant Sci., 11, 28 (2020). DOI: 10.3389/fpls.2020.00028.
- Raturi , Sharma Y., Rana V., Thakral V., Myaka B., Salvi P., Singh M., Dhar H. and Deshmukh R. Exploration of silicate solubilizing bacteria for sustainable agriculture and silicon biogeochemical cycle. Plant Physiol. Biochem., 166, 827-838 (2021). DOI: 10.1016/j.plaphy.2021.06.039.
- Lambert J.B., Gurusamy-Thangavelu S.A. and Ma K. The silicate-mediated formose reaction: bottom- up synthesis of sugar Science., 327 (5968), 984-986 (2010). DOI: 10.1126/science.1182669.
- Yachkula A. A., Yatskiv A. A., Bykov A. G., Abashina T. N., and Vainshtein M. B. Application of exopolysaccharides of bacteria of the genus Paenibacillus for bioleaching of silicate ores. In XXXIII Winter International Youth Scientific School “Perspective directions of physical and chemical biology and biotechnology”, Moscow, February 8-11, Abstract 189. (Moscow, IBCh RAS, 2021), 256 p.
- Artyszak A. Effect of silicon fertilization on crop yield quantity and quality. Plants., 87, 1-17 (2018). DOI: 10.3390/plants7030054.
- Ibrahim , Iqbal M., Tang Y.T., Khan S., Guan D.X. and Li G. Phosphorus mobilization in plant–soil environments and inspired strategies for managing phosphorus: A Review. Agronomy., 12, 2539. DOI: 10.3390/agronomy12102539.
- Lv , Li J., Ye H., Du D., Li J., Sun P., Ma M. and Wen J. Bioleaching behaviors of silicon and metals in electrolytic manganese residue using silicate bacteria. J. Clean. Product., 228, 901-909 (2019). DOI: 10.1016/j.jclepro.2019.04.289.
- Tserkovniak L.S. and Kurdish I.K. Phosphate-mobilizing bacteria Bacillus subtilis as phenolic producers. Biochem. Microbiol., 45, 279–284 (2009). DOI: 10.1134/S0003683809030077.
- Shwethakumari U. and Prakash N.B. Effect of foliar application of silicic acid on soy bean yield and seed quality under field conditions. J. Ind. Soc. Soil Sci., 66 (4), 406-414 (2018). DOI:10.5958/0974- 2018.00051.8.
- Liang , Sun W., Zhu Y.G. and Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ. Pollution., 54, 422-428 (2007). DOI: 10.1016/j.envpol.2006.06.008.
- Miyake Y. and Takahashi E. Effect of silicon on the growth of solution-cultured cucumber plant. Soil Plant Nutr., 56, 71-83 (1993). DOI: 10.1080/00380768.1983.10432407.
- Belogolova А., Sokolova M.G., Gordeeva О.N. and Vaishlya О.B. Speciation of arsenic and its accumulation by plants from rhizosphere soils under the influence of Azotobacter and Bacillus bacteria. J. Geochem. Explor., 149, 52-58 (2015). DOI: 10.1016/j.gexplo.2014.11.017.
License
Copyright (c) 2025 Ольга Борисовна Вайшля, Екатерина Борисовна Кудряшова, Елена Викторовна Арискина, Татьяна Николаевна Абашина, Михаил Борисович Вайнштейн (Автор)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.